We will now take a look at the energy budget for the planet Earth and provide an explanation for the greenhouse effect. A detailed study of this material is too complex to cover in this class. However, I will attempt to go into enough detail to allow you to understand the basics. We will then apply this material to study global warming issue.
We must first briefly describe some of the properties of radiation and the natural emission of radiation.
Radiation is a form of energy transfer. Radiation energy emitted (or given off) by one object is delivered to another object when that object absorbs the radiation energy. Radiation energy travels through outer space at the "speed of light" (300,000 km/sec or 186,000 mi/hr). Visible light is just one type or classification for radiation. It is the type of radiation that can be sensed by our eyes. We cannot see other types of radiation, but they exist.
All objects in the universe emit (or give off) radiation. At a very basic level, radiation is emitted photon by photon. Billions upon billions upon billions ... of photons. Different types of radiation can be described by the energy carried by a single photon. As mentioned previously, for studies of the Earth's climate, we are mainly concerned about ultraviolet, visible, and infrared radiation (listed in order from most energetic to least energetic photons). Keep in mind that there exists a continuous spectrum of photon energies in the universe, which extends from much more energetic than ultraviolet to much less energetic than infrared.
For solids, liquids, and high density gases (like stars), we can characterize the radiation emitted using the following two rules:
More specifically the rules above can be written as mathematical equations, that is, given the temperature of an object, the rate of photon emission over the entire range of photon energies can be computed. We can also work backwards: by measuring the radiation energy emitted by an object, we can determine its temperature. For example, if I point an infrared detector toward you, I will measure radiation characteristic of a 98°F object. If I point the detector at a colder wall in the room, I will measure less radiation energy, which is the amount characteristic of an object with a temperature of about 75°F. Because the infrared detector is measuring radiation emitted by objects on Earth (rather than normal vision which works by seeing visible light that is reflected from an object), the infrared detector can sense the difference between you and the cold wall even in the dark. This is how military night vision works. The presence of people (at night) can be detected by measuring the higher amount of infrared energy emitted by warm bodies compared to colder surroundings. Differences in the infrared energy measured are then displayed as a false color visible images that can be seen by human eyes. The two figures below are false color infrared images. One before the boy bounces a ball and one after the ball has been bounced. Because the ball gets hotter after being bounced it emits more infrared radiation. The scale on the right relates the displayed false color with temperature. Similarly, the temperature of the Earth can be determined by satellite measurements of infrared radiation emitted from the surface of the Earth and the atmosphere.
 |
 |
The actual energy transfer takes place when an object absorbs the radiation energy that was emitted by another object. Absorption can be said to occur photon by photon. Once a photon is absorbed, it ceases to exist, and its energy is delivered to that object. In the case of the Earth and Sun, the Earth heats up when it absorbs radiation energy emitted from the Sun.
Radiation is the only form of energy transfer that can move through the vacuum of outer space. Thus, radiation is the only way for energy to be transfered into or away from the planet Earth. The original source of nearly all of the energy for the physical and biological processes that take place on Earth is radiation energy that came from the Sun. The Earth continuously emits radiation energy off to space. The net effect of these two processes (absorption of radiation energy from the Sun and emission of radiation energy to space) determines the average temperature for the planet.
Because the Sun is much hotter than the Earth, it emits much more radiation energy than the Earth. Since the temperature of the Sun is about 5800 K, the majority of the radiation energy it emits is in the form of visible radiation. Similarly, since the temperature of the Earth is about 288 K, the majority of the radiation energy it emits is in the form of infrared radiation. So the Earth heats up by absorbing mostly visible radiation energy from the Sun (with smaller amounts of energy in the form of ultraviolet and infrared radiation), and cools down by emitting infrared radiation out to space. Over time these two processes come into balance, i.e., radiation energy absorbed equalling radiation energy emitted, and the planet Earth reaches a steady average temperature (as described below):
 |
If there were no atmosphere, the average temperature of the Earth's surface would be equal to the radiative equilibrium temperature of 0°F. But the atmosphere acts to warm the surface through what is called the atmospheric greenhouse effect. The average surface temperature of the Earth is about 59°F. We will explain more about the greenhouse effect later. You should understand that the average temperature at the Earth's surface does not have to be equal to the average temperature of the planet as a whole. For instance, we know that the temperature of the atmosphere near the tropopause is much colder than the temperature at the Earth's surface.
Before describing the greenhouse effect, let us take a look at what happens to the radiation from the Sun that hits the planet Earth. Included in these notes are explanation for why the sky looks blue, why sunsets are red, why clouds are white, and how haze affects visibility.
To determine the average temperature of the planet Earth, what is important is how much radiation energy from the Sun is absorbed by the planet. The amount of radiation energy that hits the planet can be calculated using the temperature of the Sun, the distance from the Sun to the Earth and the size of the Earth. However, not all of this radiation is absorbed. Some is scattered back to space. Only the absorbed radiation goes into heating the Earth. The following two figures illustrate this pictorially.
| We will follow a beam of solar energy and study its
interaction with the atmosphere and the Earth's surface.
Assume the beam contains 100 units of energy as it enters the atmosphere: |
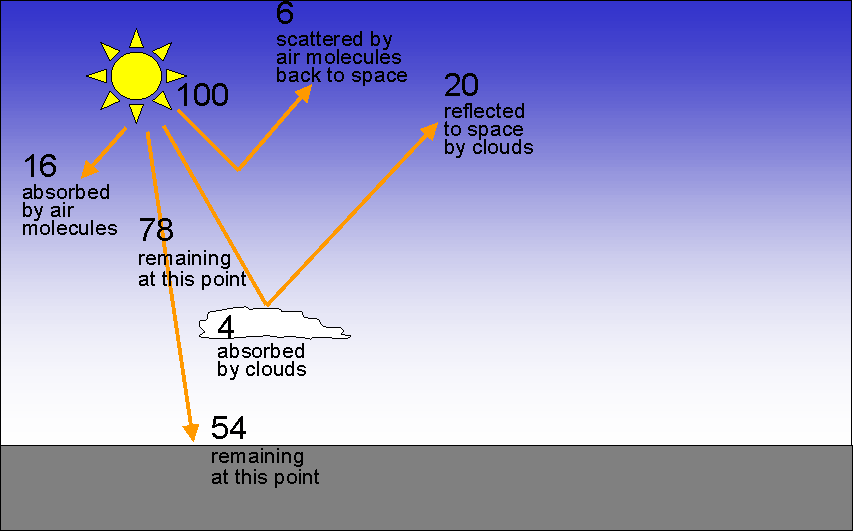
|
| On a worldwide, long-term average, air molecules absorb 16% of the incoming energy from the Sun and reflect another 6%. Clouds absorb 4% of the incoming energy from the Sun and reflect 20%. Therefore 54% of the incoming energy from the Sun is transmitted through the atmosphere and hits the Earth's surface. |

|
| Complete solar portion of the Earth's energy budget. 4 of the 54 units are reflected back to space. Therefore, the 50 units left must be absorbed by the Earth's surface. |
To summarize this information, of all the solar radiation that hits the planet Earth:
 |
 |
 |