Wednesday Aug. 30, 2006
The Practice Quiz is one week from today. In anticipation of that
event, a preliminary version of the Practice
Quiz Study Guide is available online. There may be some small
changes made between now and the beginning of next week.
Locations of the reviews will be added to the study guide once they are
known. Note there won't be a review next Monday afternoon; Monday
is a holiday.
The packet containing old quizzes and an old final exam is now
available for purchase ($2.50).
Tropical storm Ernesto is moving through Florida and into the SE United
States. Meanwhile in the Pacific, off the west coast of Mexico, Hurricane John has strengthened
into a category 4 hurricane. Hurricane John may influence our
weather by the end of the week.
A short new reading assignment was
made.
 This rather
busy and confusing picture just illustrates how small changes in how
air temperature changes with increasing altitude can determine whether
the atmosphere will be stable or unstable. Just for the
purposes of illustration we imagine riding a bicycle from Swan and
River Rd up a hill to Swan and Sunrise (fhe figure shows an elevation
change of 1000 ft, it is actually quite a bit less than that)
This rather
busy and confusing picture just illustrates how small changes in how
air temperature changes with increasing altitude can determine whether
the atmosphere will be stable or unstable. Just for the
purposes of illustration we imagine riding a bicycle from Swan and
River Rd up a hill to Swan and Sunrise (fhe figure shows an elevation
change of 1000 ft, it is actually quite a bit less than that)
At far left the air temperature drops 6o F. This is a fairly
rapid drop with increasing altitude and would make the atmosphere
absolutely unstable. The atmosphere wouldn't remain this
way. Air at the ground would rise, air above would sink, and the
temperature profile would change. In some ways it would be like
trying to pour vinegar on top of oil in a glass. The lower
density oil would rise because it would "want" to float on top of the
higher density vinegar.
The next picture shows air temperature decreases a little more slowly
with increasing altitude. This small change makes the atmosphere
conditionally unstable (we won't go into the conditions). The
atmosphere is frequently in this state.
The atmosphere cools only 2o F in the next picture. This creates
an absolutely stable atmosphere. Air at the ground will remain at
the ground and won't rise and mix with air higher up. Compare
this with the glass containing vinegar and a layer of oil on top.
The two layers won't mix.
Air temperature in the last figure actually increases with increasing
altitude This is a temperature inversion and produces very
stable conditions.
Sulfur
dioxide is the next of the air pollutants that we will discuss.

Sulfur dioxide is produced by the combustion of sulfur
containing
fuels such as coal. Combustion of fuel also produces carbon
dioxide and carbon monoxide. People probably first became aware
of sulfur dioxide because it has an unpleasant smell. Carbon
dioxide and carbon monoxide are odorless.

The Great London smog is still the deadliest air pollution
event in
history. Because the atmosphere was stable, SO2 emitted into air
at ground level couldn't mix with cleaner air above. The SO2
concentration was able to build to dangerous levels.
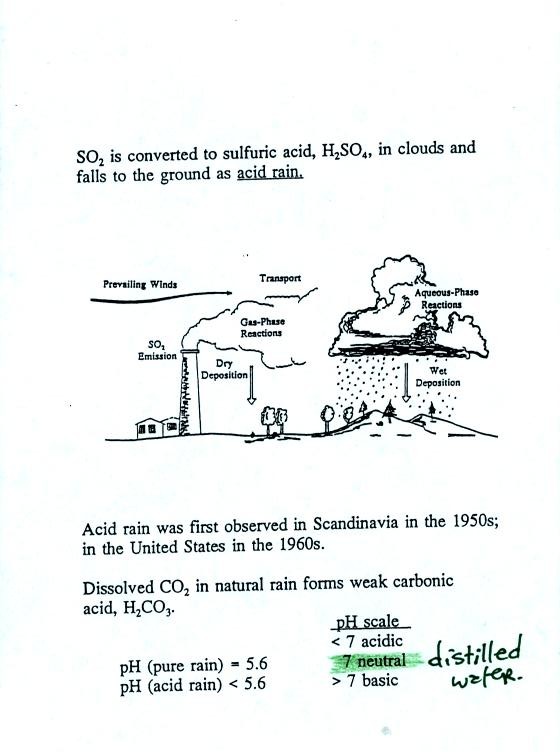
Acid rain often falls hundreds or
thousands of miles away from the
source of the SO2. Coal fired factories and electric
power plants
in the Ohio River Valley could produce acid rain in New England and
Canada. Acid rain in Scandinavia could be the result of SO2
emissions in England and Belgium. Oxides of nitrogen (NO, NO2,
and N2O) also react in clouds to form acid rain (nitric acid).

An acid rain demonstration was
performed in class to give you a general idea of how acid rain is
produced. Carbon dioxide rather than SO2 was bubbled
through
Tucson tap water. The tap water is initially slightly basic (pH
> 7). Dissolved CO2 however turned the tap water
acidic.
We will discuss tropospheric ozone, a pollutant, in class on
Friday. It is important to understand that ozone is found in the
stratosphere where it is beneficial. It is the ozone that is
found in the troposphere that is considered a pollutant and is one of
the key ingredients in photochemical smog.
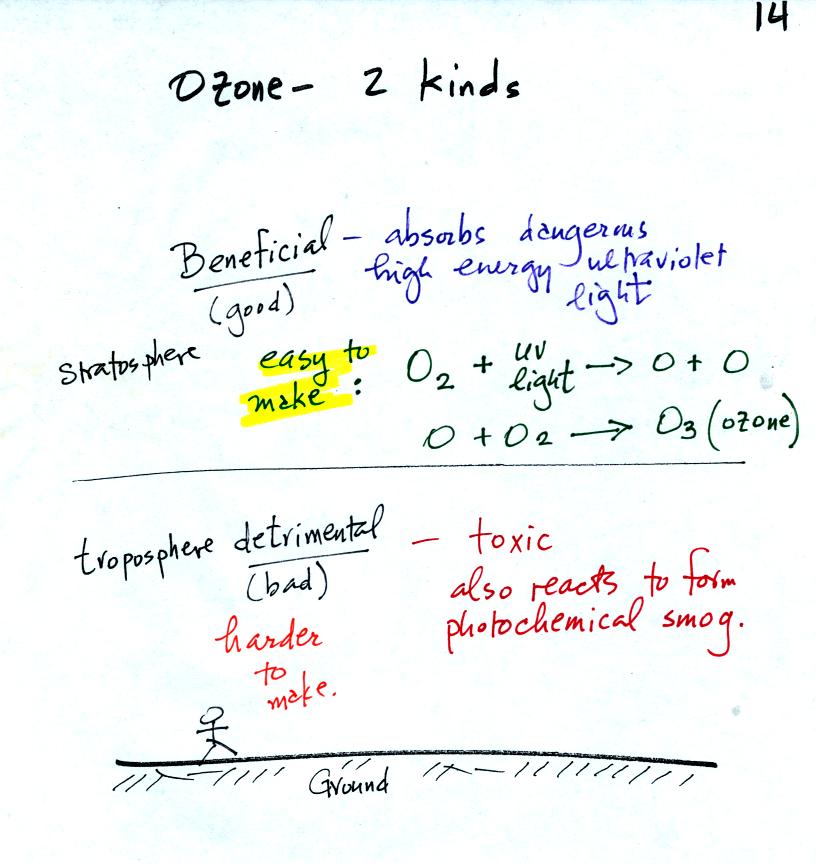
It is relatively easy to make ozone in the statosphere. We
will make use of this simple two step reaction in class on
Friday. We will see that ozone production in the troposphere is a
little more complicated.
 This rather
busy and confusing picture just illustrates how small changes in how
air temperature changes with increasing altitude can determine whether
the atmosphere will be stable or unstable. Just for the
purposes of illustration we imagine riding a bicycle from Swan and
River Rd up a hill to Swan and Sunrise (fhe figure shows an elevation
change of 1000 ft, it is actually quite a bit less than that)
This rather
busy and confusing picture just illustrates how small changes in how
air temperature changes with increasing altitude can determine whether
the atmosphere will be stable or unstable. Just for the
purposes of illustration we imagine riding a bicycle from Swan and
River Rd up a hill to Swan and Sunrise (fhe figure shows an elevation
change of 1000 ft, it is actually quite a bit less than that)



