Friday, Sept. 1, 2006
A copy of the Practice Quiz Study Guide was handed out in class.
Hurricane John is near the southern tip of Baja California. The National
Hurricane Center is currently predicting it will travel along the
west coast of Baja California and then turn toward the west. Some
models are predicting paths that would carry the storm and remnants
of the storm into the Gulf of California and even into the western edge
of Arizona. There is a chance that moisture from Hurricane John
could move into Arizona and produce heavy rain and flooding. The
chances of this occurring are very uncertain at the present time.
You can keep track of the latest weather advisories at the Tucson National Weather Service
web page.
Tropospheric
ozone is a pollutant. Tropospheric ozone is also a key component
of photochemical smog.
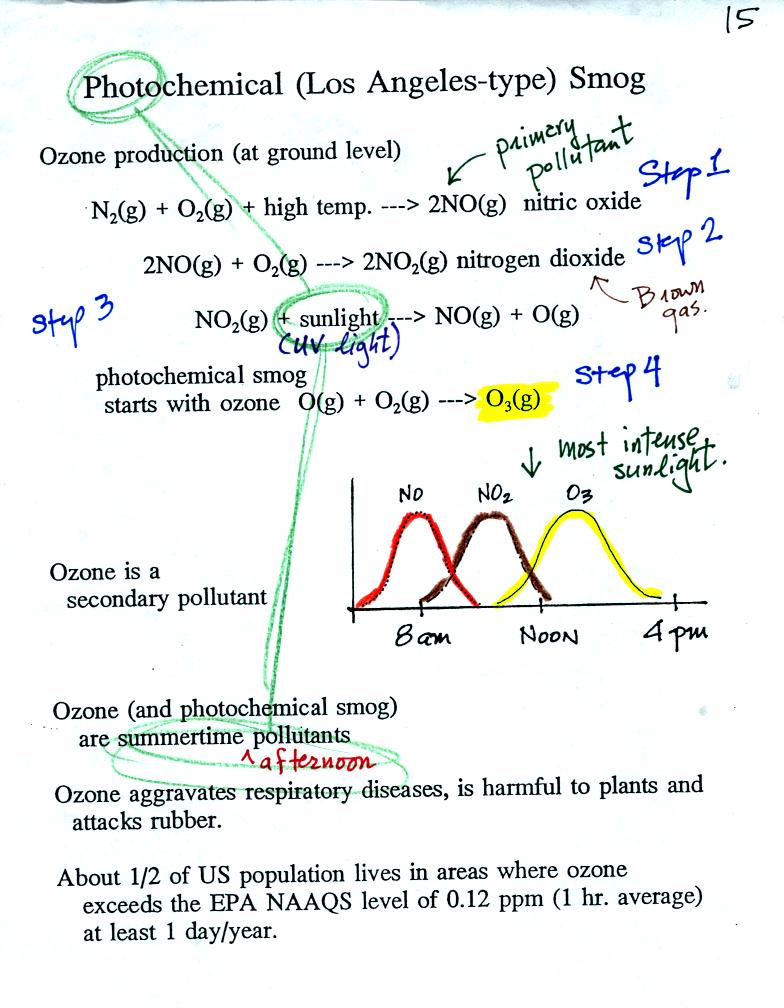
The production of tropospheric
ozone begins with nitric
oxide
(NO). NO is produced when nitrogen and oxygen are heated (in an
automobile engine for exampe) and react. The NO can then react
with oxygen to make nitrogen dioxide, a poisonous brown-colored
gas. Sunlight can dissociate (split) the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2
react (just
as they do in the stratosphere) to make ozone (O3).
Because ozone
does not come directly from an automobile tailpipe or factory chimney,
but only shows up after a series of reactions, it is a secondary
pollutant. The nitric oxide would be an example of a
primary pollutant.
NO is produced early in the day. The concentration of NO2
peaks
somewhat later. Peak ozone concentrations are usually found in
the afternoon. Ozone concentrations are also usually higher in
the summer than in the winter. This is because sunlight plays a
role in ozone production and summer sunlight is more intense than
winter sunlight.
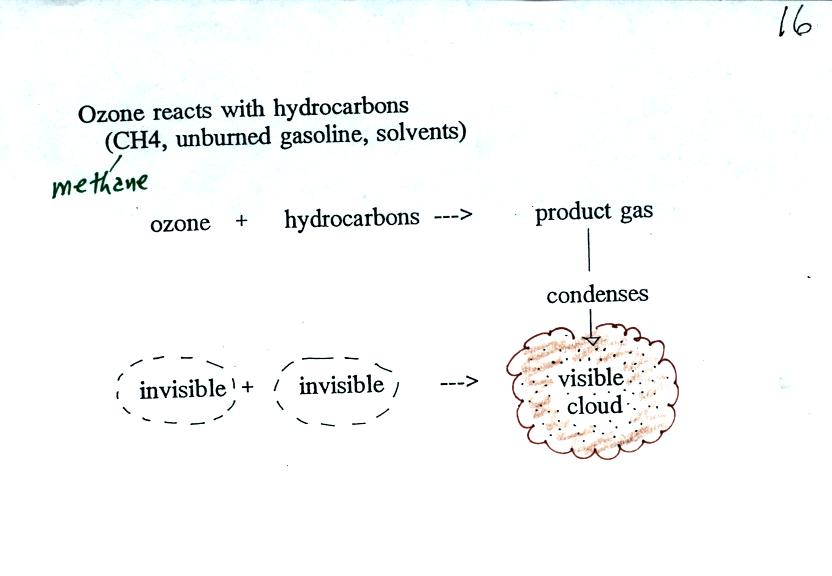
As shown in the figure below,
invisible ozone can react with a hydrocarbon of some kind which is also
invisible to make a
product
gas. This product gas sometimes condenses to make a visible smog
cloud or haze.
The class demonstration of photochemical smog is summarized
below (a flash was used instead of the aquarium shown on the bottom of
p. 16 in the photocopied class notes). We begin by using the UV
lamp to fill the flask with
ozone. Then a few pieces of fresh lemon peel were added to the
flask. A whitish cloud quickly became visible (colored brown in
the figure below).

For the remainder of today's class and also next Friday
(after the Practice Quiz on Wednesday) we return to
the middle part of Chapter 1 and will look at how characteristics such
as air temperature, pressure, and density vary with changing altitude
in the atmosphere. We'll start with temperature.
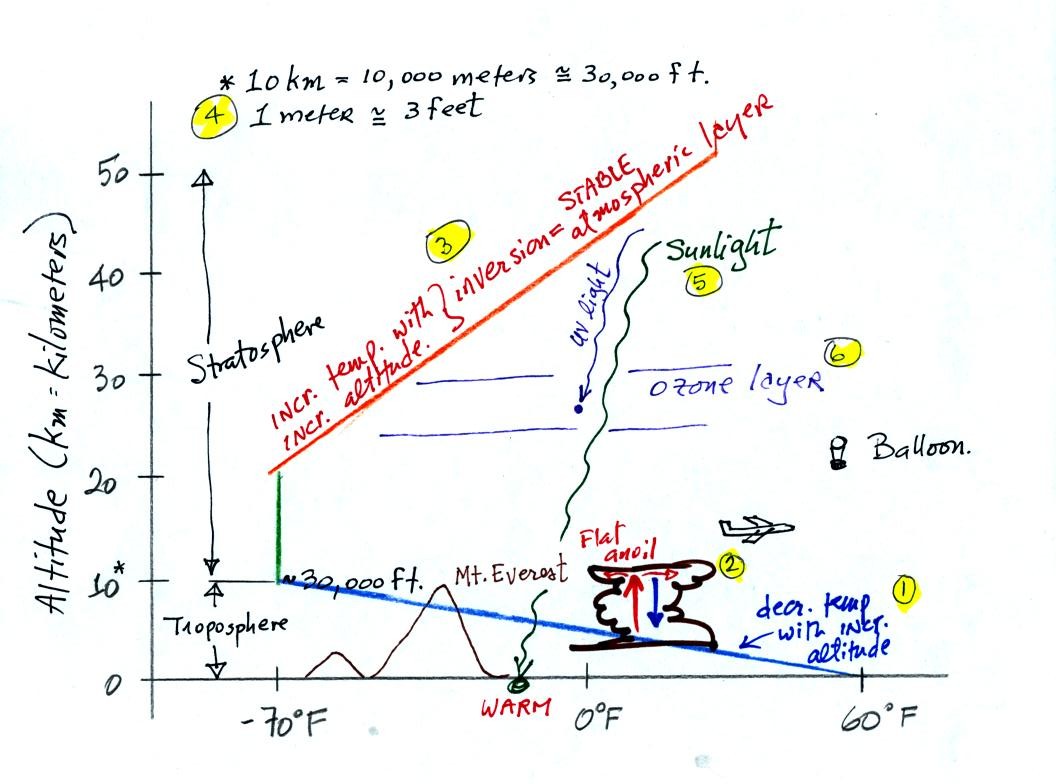
The atmosphere can be split
into layers
depending on whether
temperature is increasing or decreasing with increasing altitude.
The two lowest layers are shown in the figure above. (the numbers
1 - 6 were added after class to aid the discussion of this figure)
1. We live in
the troposphere. The troposphere is found, on average, between 0
and about 10 km altitude, and is where temperature using decreases with
increasing altitude.
Most of the sunlight arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground. This warms the ground. The air in contact with the
ground is warmer than air higher up and further from the ground.
2. The troposphere contains most of the water vapor
in the atmosphere and is where most of the weather occurs. The
troposphere can be stable or unstable (tropo means to turn over and
refers to the fact that air can move up and down in the
troposphere). The thunderstorm shown in
the figure indicates unstable conditions, meaning that strong up and
down air motions are occurring. When the thunderstorm reaches the
top of the troposphere, it runs into the stable stratosphere. The
air can't continue to rise in the stable stratosphere so the cloud
flattens out and forms an anvil.
3. Temperature remains constant between 10 and 20 km and then
increases with increasing altitude between 20 and 50 km. These
two sections comprise the stratosphere. The stratosphere is a
very stable air layer.
4. 10 km (kilometers) is approximately 30,000. At
nearly 30,000 feet altitude, the summit of Mt.
Everest is near the top of the troposphere. Commercial aircraft
fly at cruising altitudes between 30,000 and 40,000 feet. This is
right at the boundary between the top of the troposphere and the bottom
of the stratosphere.
5. Most of the sunlight arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground. This warms the ground. The air in contact with the
ground is warmer than air higher up and further from the ground (in the
troposphere anyway).
6. How do you explain increasing temperature with increasing
altitude in the stratosphere. The ozone layer is found in the
stratosphere (peak concentrations are found near 25 km altitude).
Absorption of
ultraviolet light by ozone warms the air in the stratosphere and
explains why the air can warm. The air in the stratosphere is
much less dense (thinner) than in the troposphere. It doesn't
take as much energy to warm this thin air as it would to warm denser
air closer to the ground.
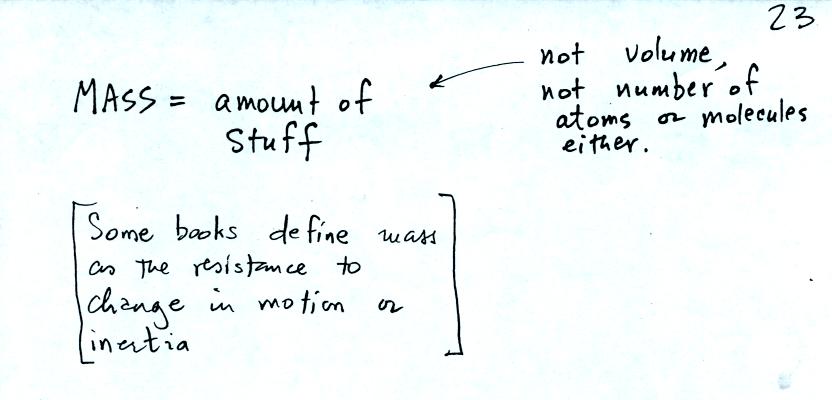
Before we can learn about atmospheric pressure, we need to review
the terms mass and weight. In some textbooks you'll find mass
defined at the "amount of stuff." Other books will define mass as
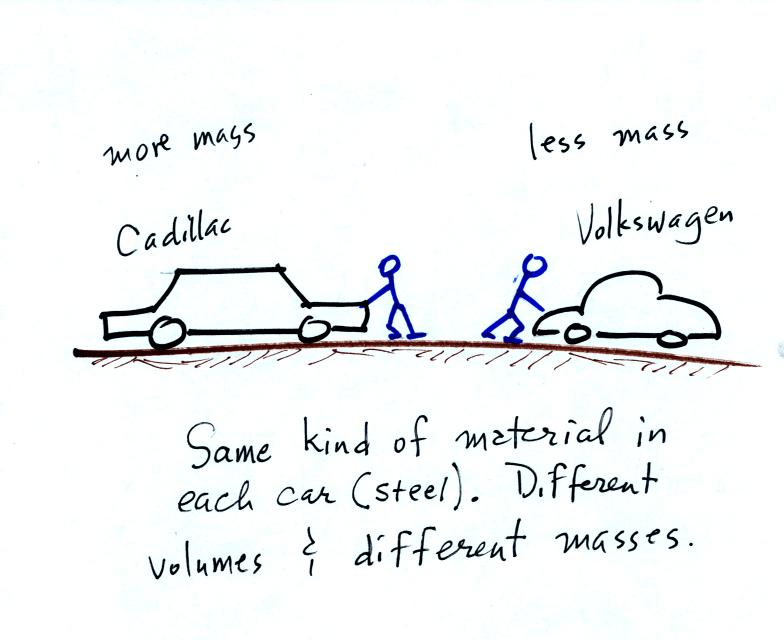
inertia or as resistance to change in motion. The next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving, it has
a larger inertia (it would also be harder to slow down if it were
already moving).

We tend to use weight and mass interchangeably because we spend all our
lives on earth where gravity never changes. Next we can explore
the concept of mass a little more by considering two equal volumes of
different materials.
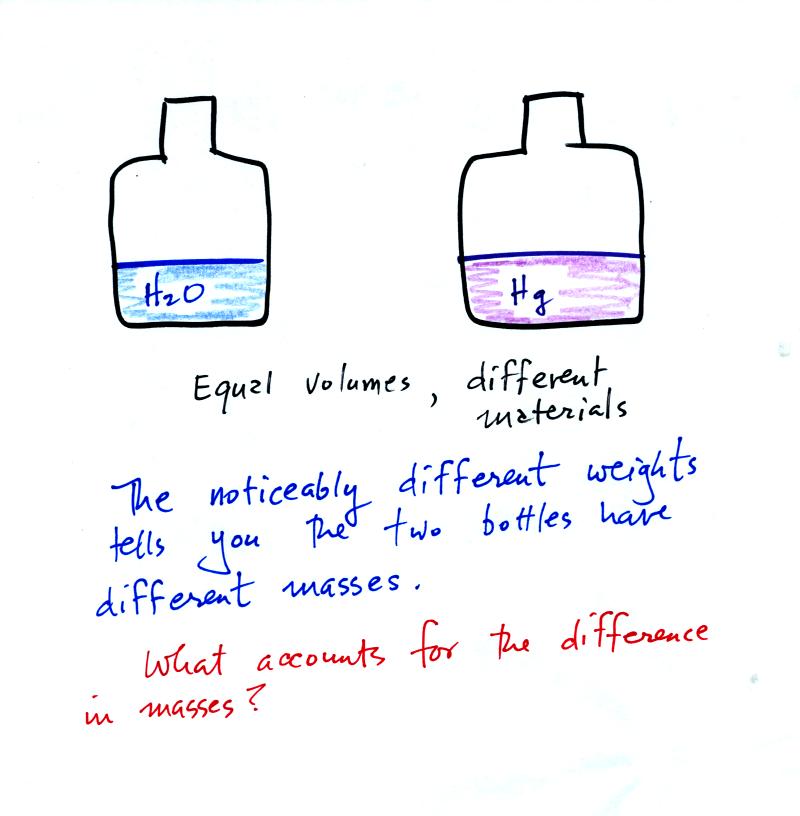
One student suggested that the bottle of mercury above (bottles of
water and mercury will be passed around in class next Friday) might
contain more atoms than the bottle of water even though the volumes are
the same. We will see that this is actually true. But even
if the two bottles contained the same volumes and the same numbers of
atoms or molecules of water and mercury, the masses would be very
different. There is another more basic difference between water
and mercury.










