Wednesday Sept. 13, 2006
The Quiz #1 Study Guide (in preliminary
form) is now available online.
A new reading section has been
assigned.
Here's a
reminder of what we will be covering today in an effort to understand
why warm air rises and cold air sinks.
An
explanation or discussion of these figures has been
purposely left off. By the end of the class today (and after some
review of your notes), you should understand and be able to explain
what is being depicted.
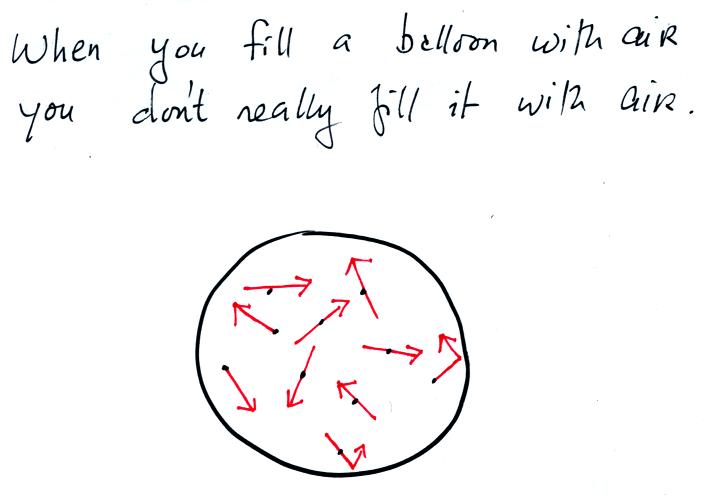
Collisions between the air atoms or molecules and the sides of the
balloon keep the balloon inflated.
The ideal gas law equations tell you how variables like
the number of gas molecules,
the volume of the balloon, and
the density and temperature of the air
affect the pressure of the air in the balloon.
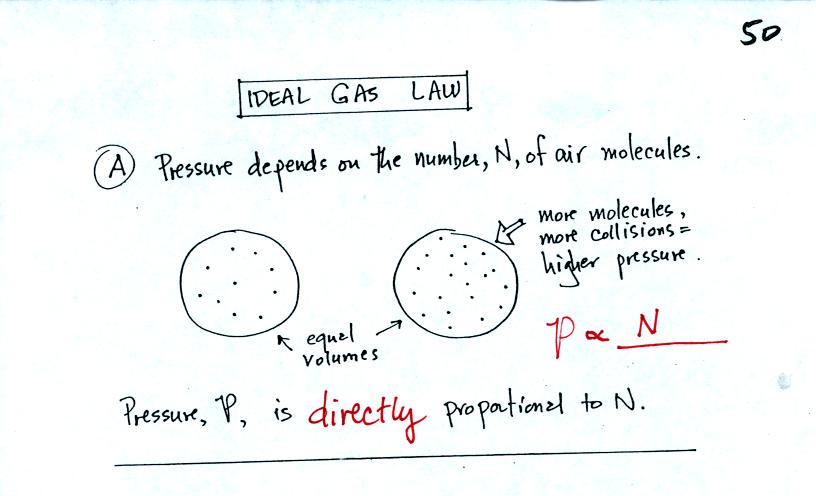
The pressure produced by the air molecules inside a balloon will
first depend on how many air molecules are there.
As you add more and more add to something like a bicycle tire, the
pressure increases.
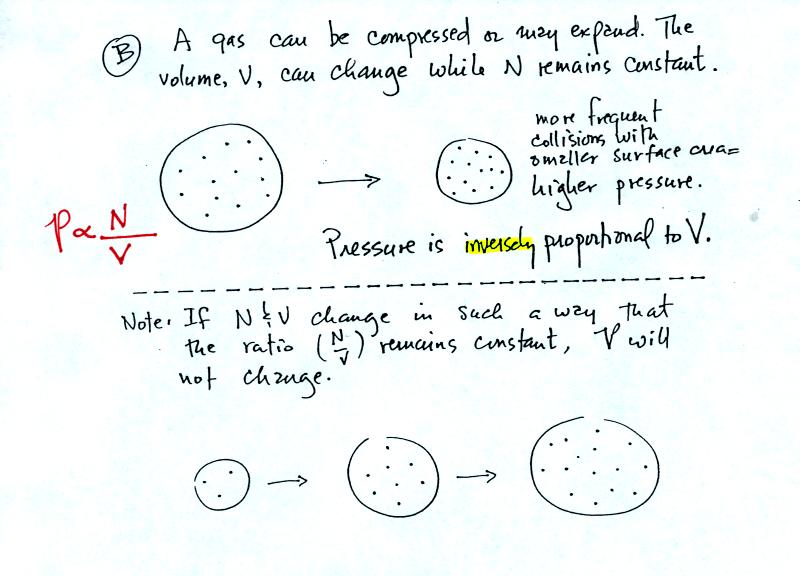
Air pressure inside a balloon also
depends on the size of the
balloon. Pressure is inversely proportional to volume, V
(increasing V decreases P and vice versa).
Note it is possible to keep pressure constant by changing N and V
together in just the right kind of way. This is what happens in
Experiment #1 that some of you are working on. Water is able to
move into the inverted cylinder and will do so to keep the air sample
pressure equal to the pressure outside the cylinder. As oxygen is
removed from an air sample, water moves into the cylinder, the air
sample volume decreases and
the pressure of the air sample stays constant.

You
shouldn't throw a can of spray paint into a fire. The
pressure of the gas inside a container depends on the gas
temperature. If the can gets hot enough, the buildup in pressure
could cause the can to rupture.
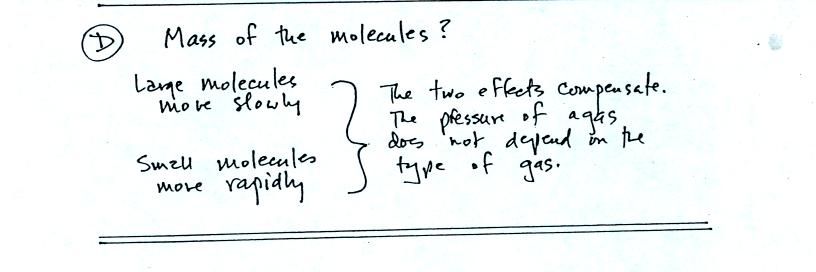
Surprisingly the pressure does
not depend on the mass of the
molecules. Pressure doesn't depend on the composition of the
gas. Gas molecules with a lot of mass will move slowly, the less
massive molecules will move more quickly. They both will collide
with the walls of the container with the same force.
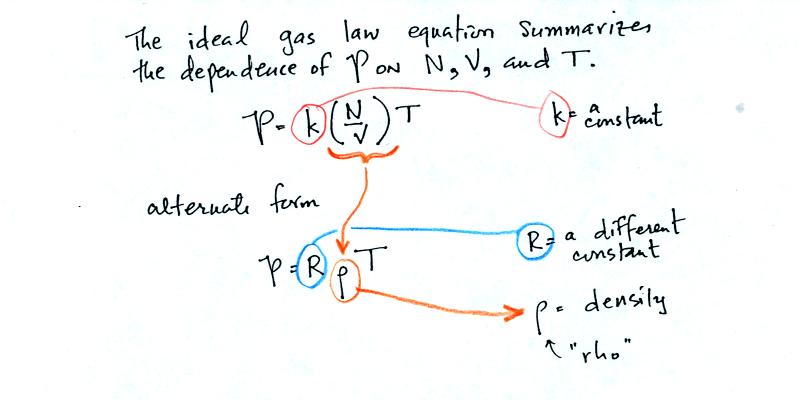
Here are the two ideal gas law equations. You can
ignore the
constants k and R if you are just trying to understand how a change in
one of the variables would affect the pressure. You only need the
constants when you are doing a calculation involving numbers.
(1) Pressure = (Number of air molecules) multiplied by temperature divided by volume
or
(2) Pressure = (density) multiplied
by (temperature)
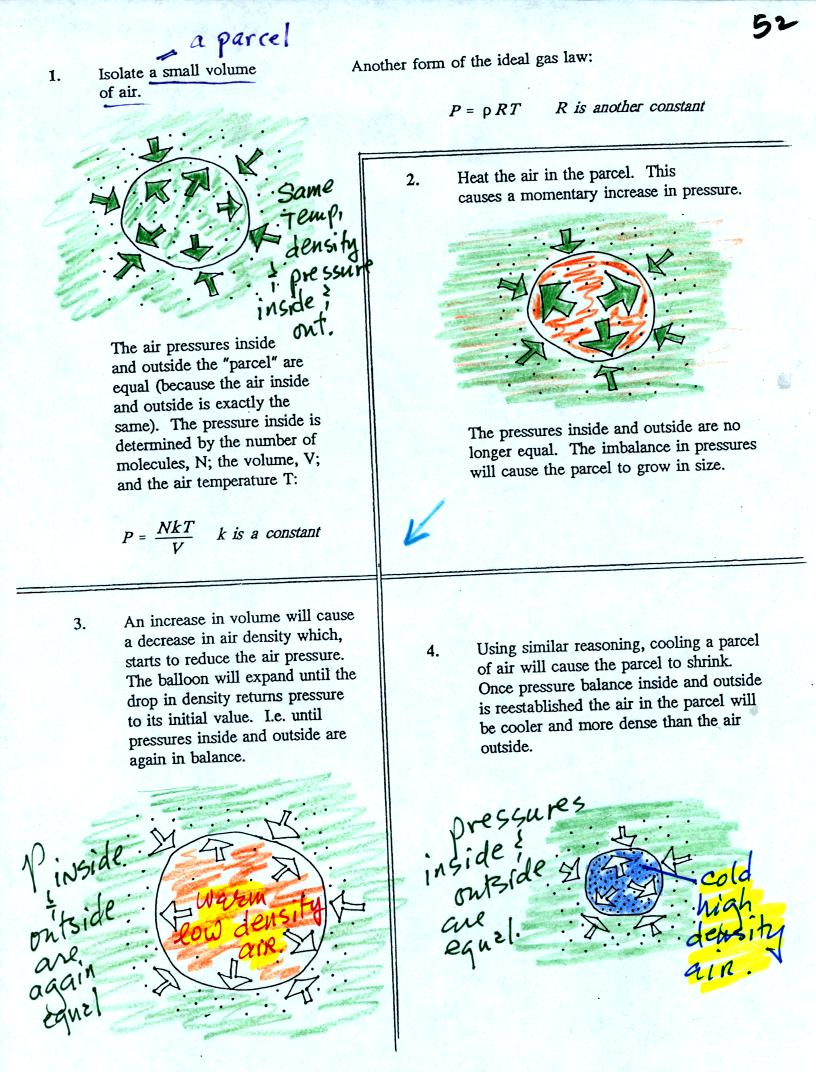
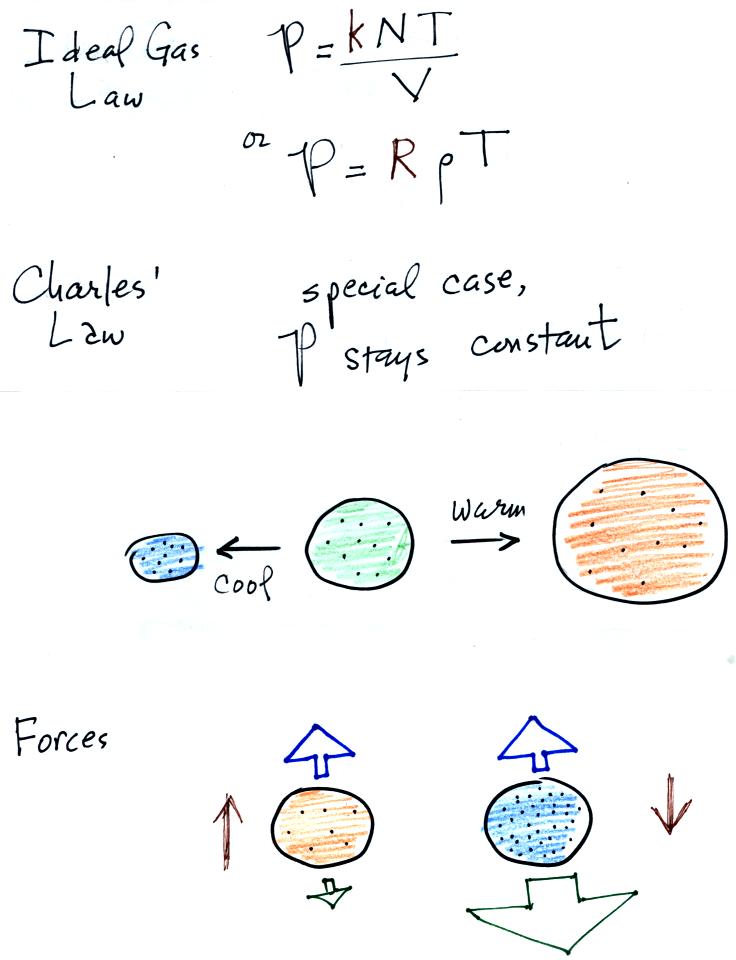
Air in the atmosphere behaves like air in a balloon. A
balloon can grow or shrink in size depending on the pressure of the air
inside.
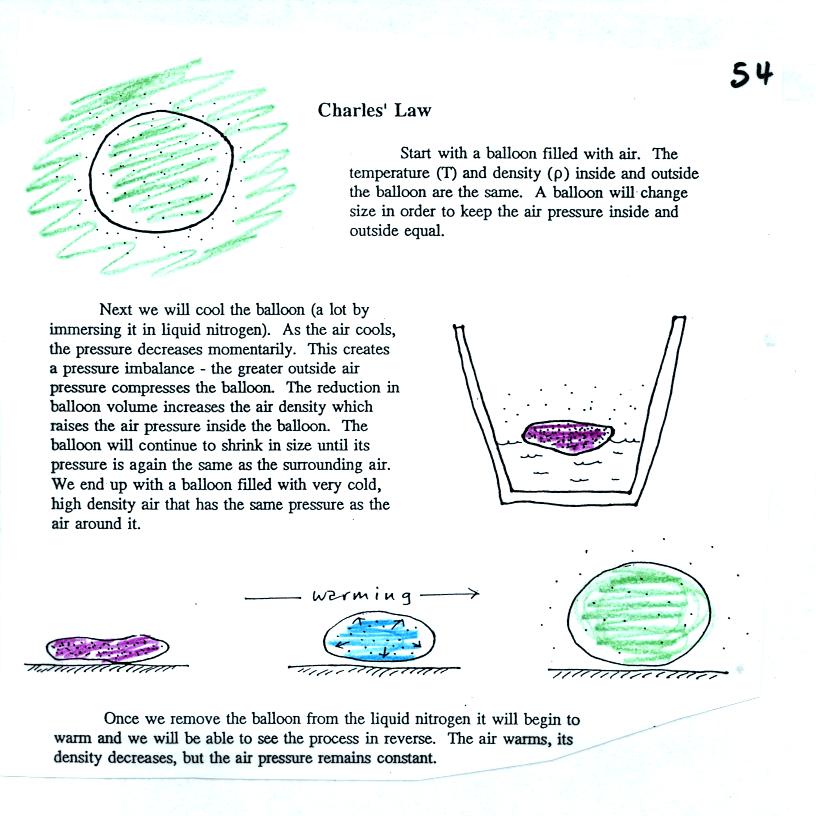
We start in the upper left hand corner with air inside a balloon that
is exactly the same as the air outside. The air inside and
outside have been colored green. The arrows show that the
pressure of the air inside pushing outward and the pressure of
the air surrounding the balloon pushing inward are all the same.
Next week warm the air in the balloon (Fig. 2). The ideal gas law
equation
tells us that the pressure of the air
in the balloon will increase. The increase is
momentary though.
Because the pressure inside is now greater than
the pressure outside, the balloon will expand. An increase in
volume will reduce the pressure of the air inside.
Eventually the balloon will expand just enough that the pressures
inside and
outside are again in balance. You end up with a balloon of warm
low density air that has the same pressure as the air surrounding it
(Fig. 3)
You can use the same reasoning to understand that cooling a balloon
will cause its volume to decrease. You will end up with a balloon
filled with cold high density air. The pressures inside and
outside the balloon will be the same.
These associations: warm air
= low density air and cold
air = high density air are important and
will come up a lot during the remainder of the semester.
In the
atmosphere air temperature and air density change together in a way
that keeps pressure constant. This is Charles's Law and was
demonstrated in class. The demonstration is
illlustrated and described at the top of p. 54 in the photocopied
notes.
We
will now look at the forces acting on a
parcel or balloon of air.
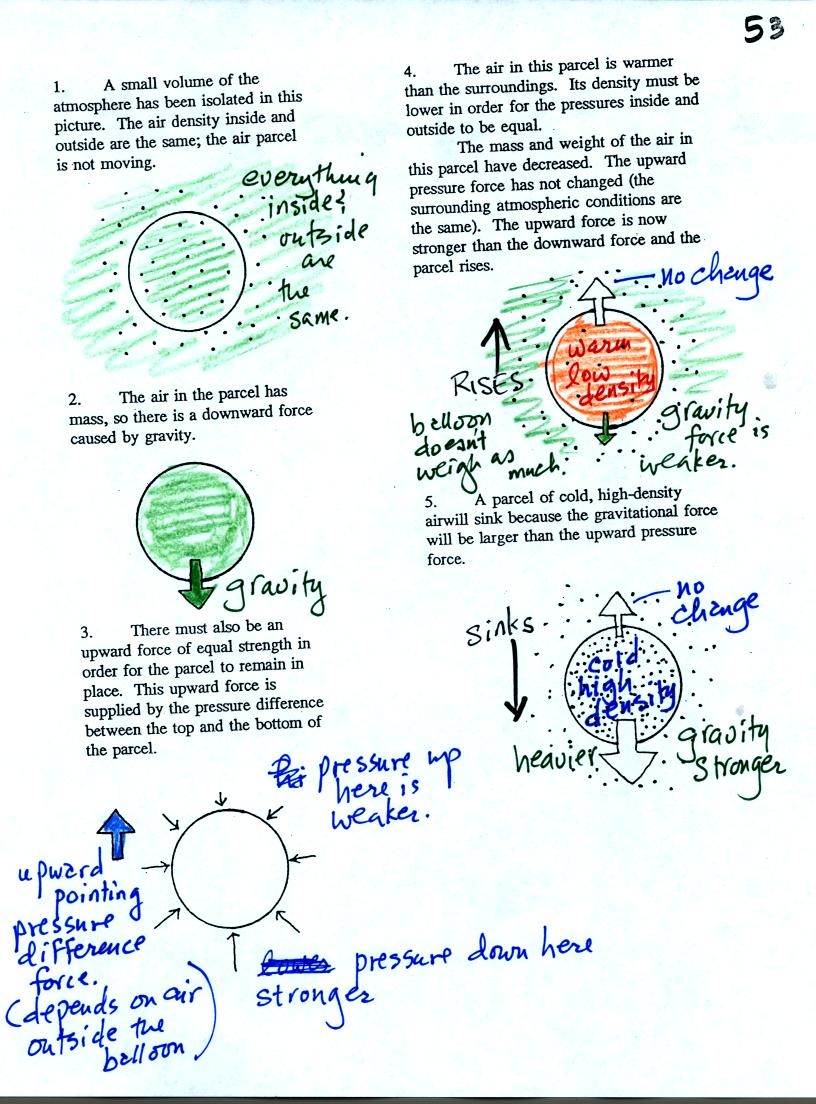
Air has mass and weight When an air parcel has the same
temperature, pressure, and density as the air around it, the parcel
will remain stationary. With gravity pulling downward on the air,
there must be another force pointing upward of equal strength.
The upward force is caused by pressure differences between the bottom
(higher pressure pushing up) and top of the balloon (slightly lower
pressure pushing down on the balloon).
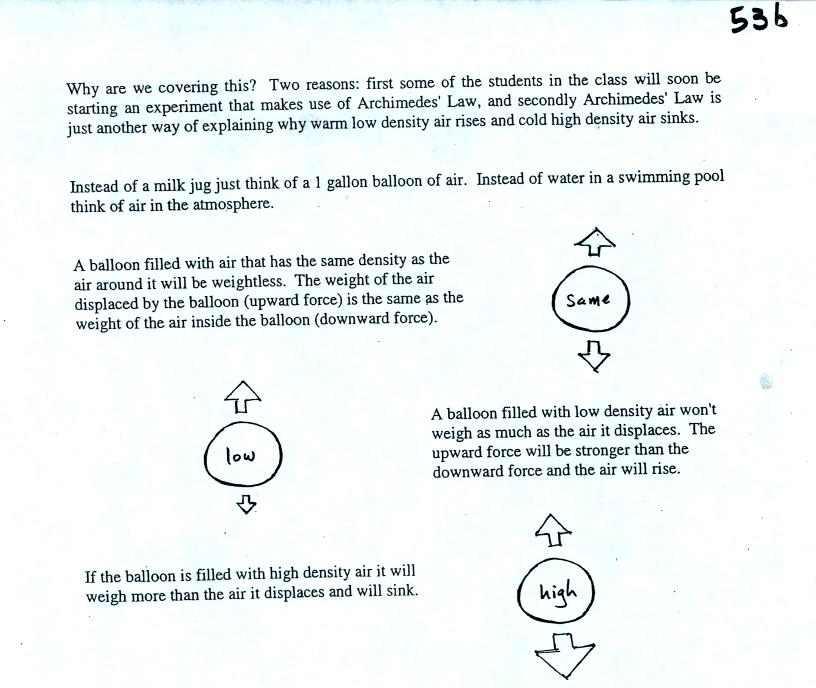
If the balloon is filled with warm, low density air the gravity force
will weaken (there is less air in the balloon so it weighs less). The
upward pressure difference force (which depends on the
surrounding air) will not change. The upward force will be
stronger than the downward force and the balloon will rise.
Conversely if a balloon is filled with cold low density air, gravity
will strengthen and the balloon will sink.

We modified the demonstration somewhat (see bottom of p. 54 in the
photocopied class notes). We used a balloon filled with helium
instead of air. Helium is less dense than air even when the
helium has the same temperature as the surrounding air. A helium
filled balloon doesn't need to warmed up in order to rise.
We dunked the helium filled balloon in some liquid nitrogen to cool it
and to cause the density of the helium to increase. When removed
from the liquid nitrogen the balloon can't rise, the gas inside is
denser than the surrounding air. As the balloon warms and expands
its density decreases. Eventually the balloon becomes less dense
than the surrounding air and lifts off from the table.
Incidentally, the balloons were gone by 8am the next morning at the
start of the T Th class.
A balloon
pilot can adjust the temperature (and thereby the density) of the air
inside a balloon and make the balloon rise or sink.
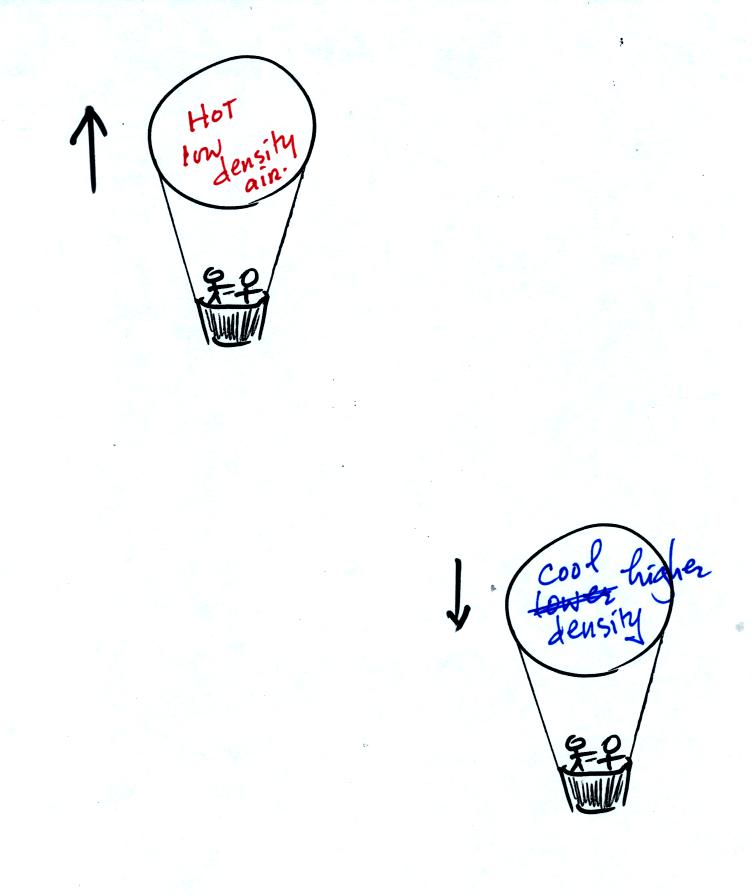
The upward
pressure difference force is really just the bouyant force in
Archimedes Law. Archimedes Law is another attempt to understand
and explain why objects float or sink. Archimedes Law is
discussed on pps 53a and 53b in the photocopied class notes. This
wasn't discussed in class.