Friday Sept. 29, 2006
There is an optional assignment due on Monday at the beginning of
class. If you want to complete the assignment but haven't picked
up a copy
you'll be able to pick one up in PAS 588 next Monday before class.
The
beginning of class was somewhat chaotic; none of the audiovisual
equipment
was working. We started covering the material on p. 45 in the
photocopied class notes.
I've taken that information and split it into two separate pages.
When you add energy to an object, the object will usually warm
up. Below we work out the equation that connects energy added and
the resulting temperature change. The equation can also be used
when energy is removed from an object. In that case the object
will cool.

When you add energy to something the temperature change will
depend on
how much energy was added. So delta E is in the numerator of the
equation. When you add equal amounts of energy to a small pan of
water and to a large pan of water, the small pan will heat up more
quickly. The temperature change, delta T, will depend on the
mass. A large mass will mean a small delta T, so mass should go
in the denominator of the equation. Different materials
react differently when energy is added to them. A material with a
large specific heat will warm more slowly than a material with a small
specific heat. Specific heat behaves in the same kind of way as
mass. Specific heat is sometimes called "thermal mass."
An object will usually warm when you add energy to it. But there
is another possibility (mentioned at the bottom of the figure).
The object could change phase. Adding energy to ice might cause
the
ice to melt. Adding energy to liquid nitrogen could cause the
nitrogen to
evaporate.

Here's an example that shows the effect of specific
heat. Equal
amounts of energy (note that calories are units of energy) are added to
equal amounts of water and dirt. We use water and dirt in the
example because most of the earth's surface is either water or dirt.
Water has a higher specific heat than soil, it only warms up 4 C.
The soil warms up 16 C.
I forgot to show the next figure
in class. Because of different specific heats, land and
water warm at different rates in the summer and cool differently in the
winter.
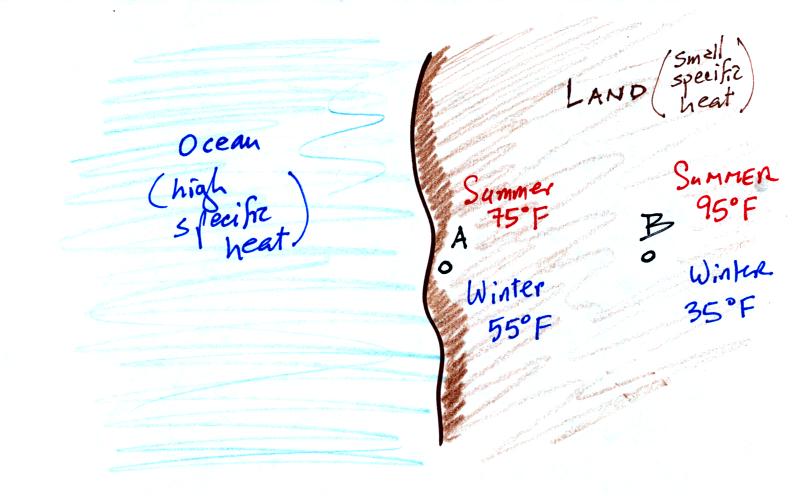
Because of its proximity to the ocean, a city on a coast
won't warm up as much during the summer and
won't cool as much during the winter as a city that is further
inland. In the example above City A has an annual range of
temperature of 20 F, in City B there is a 60 F difference between
summer and winter temperatures.
OK so you add energy to an object and the object warms. What is
that increase in temperature telling you about what is happening inside
the material?

Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material. You can think of heat as being the sum
total kinetic energy of all the molecules or atoms in a material.
The next figure might make the distinction between temperature (average
kinetic energy) and heat (total kinetic energy) clearer.
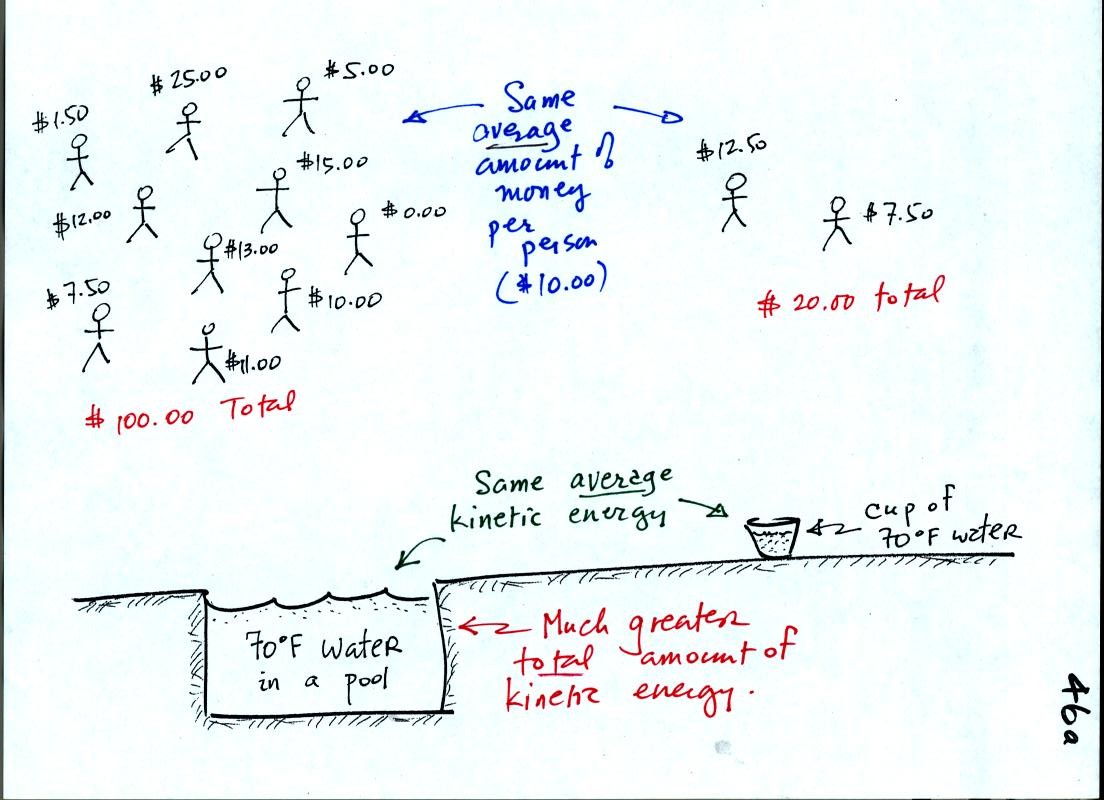
A cup of water and a pool of water both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So the total kinetic
energy of all the molecules in the pool is going to be much larger than
the total of all the kinetic energies in the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people have the same average amount
of money per person. The $100 held by the group at the left is
greater than the $20 total possessed by the smaller group of people on
the right.
You need to be careful what temperature scale you use. You must
use the Kelvin temperature scale because it does not go
below zero.

You should remember the temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and Kelvin scales. 300
K is a
good easy to remember value for the global annual average surface
temperature of the earth.

You certainly don't need to try to remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than near or
surrounded by ocean. You'll find more record high and low
temperature data on p. 58 and p. 60 in Chapter 3 of the text.
Precipitation records are shown on p. 348.
We did a
short experiment in class (it was actually done at the end of class but
I'm going to insert it in the online notes here)
The object of the experiment was to measure the latent heat of
vaporization of liquid nitrogen. That just means measuring the
amount of energy needed to evaporate a gram of liquid nitrogen.
The students that are doing Experiment #2 are measuring the latent heat
of fusion of ice, the energy needed to melt one gram of ice.
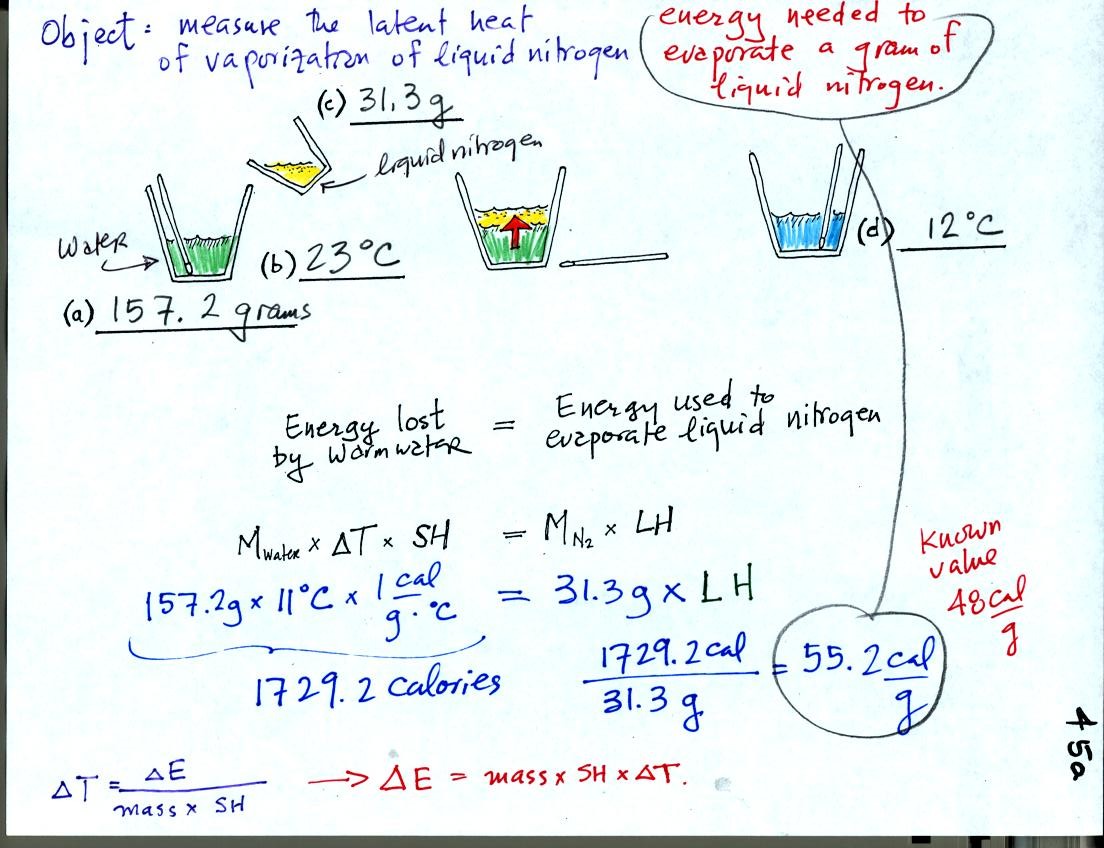
First we will make use of the Delta T vs Delta E equation
(see lower left corner in the picture above). Rather than solving
for Delta T we will
rearrange the equation and solve for Delta E.
We poured 157.2 grams of water into a styrofoam cup and measured its
temperature (23 C). Then we weighed out 31.3 grams of liquid
nitrogen into a second cup and poured it into the cup of water (after
having removed the thermometer). We waited until all the liquid
nitrogen had evaporated and remeasured the temperature of the water.
It takes energy to turn liquid nitrogen into gaseous nitrogen.
The needed energy came from the water. When energy is removed
from the water the water cools. By measuring how much the water
cooled and knowing how much water we had we can calculate how much
energy was given up by the water. That is the 1729.2 calorie
figure above. This was used to evaporate 31.3 grams of liquid
nitrogen. So 55.2 calories was needed per gram. That is our
measured value. The know value is 48 cal/g, so our measurement
was reasonably close to the known value.
There are
four energy transport processes: conduction, convection, latent heat
and electromagnetic radiation. We only had time to look at one of
these in class today.
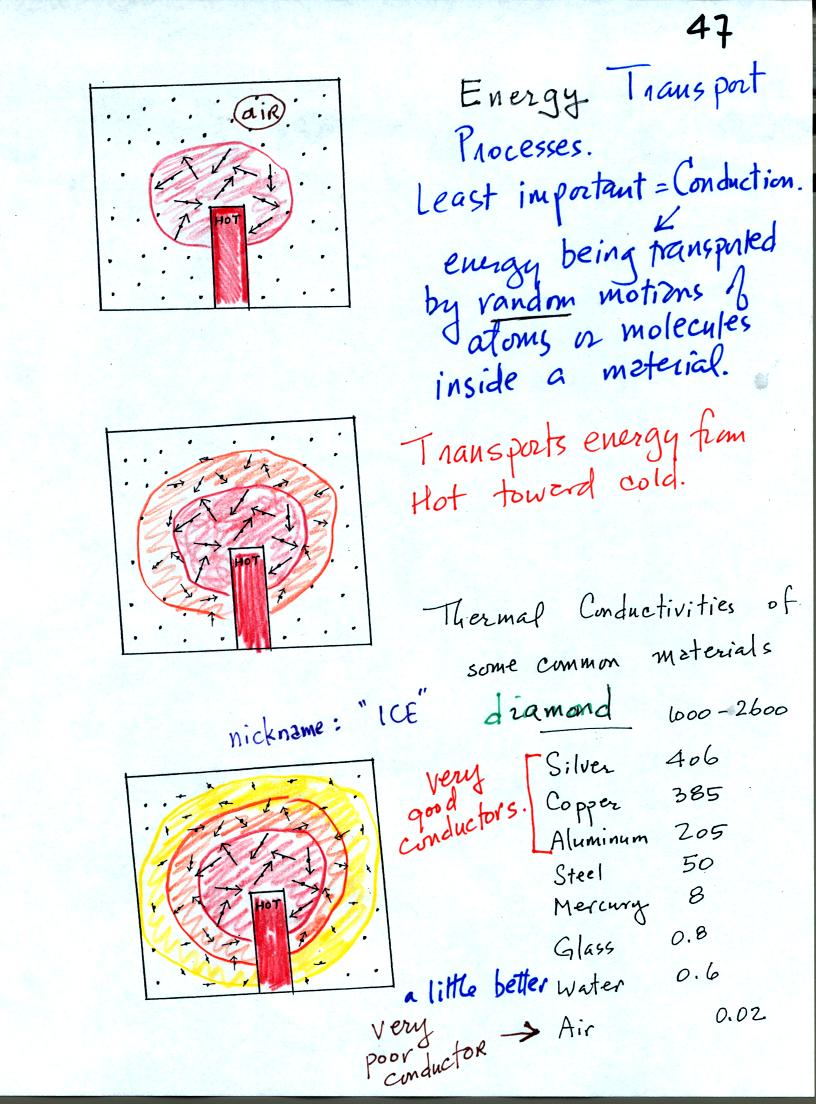
The figure above illustrates energy transport by
conduction. A
hot object is stuck in the middle of some air. In the first picture the
random motions of the atoms or
molecules near the object have caused them to collide with and pick up
energy from the object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored red). In the middle picture the
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object. In
the third picture molecules further from the object now have gained
some energy (the yellow ones). The random motions and collisions
between molecules
is carrying energy from the hot object out into the material.
The rate of energy transport depends on the material. Thermal
conductivities of some materials are listed above. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors. Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.

Here's a demonstration that we didn't do in class (this figure wasn't shown in class).
If the instructor were to open a bottle of glacial acetic acid (acetic
acid gives vinegar its characteristic smell) in the front of the
classroom, the odor would eventually spread throughout the class
room. This is an example of diffusion. The acetic acid
molecules would be moved through the room by random collisions with air
molecules. In many respects this is like the conduction of
heat. The demonstration wasn't performed because the
concentration of the acetic acid in the air, at least in the front of
the room, would be high enough to present a serious risk to the
instructor and students.









