Tuesday, Aug. 22.
The first day of class.
We first briefly discussed the Course
Information
handout. Note the various options you have for purchasing a copy
of the course textbook. You should try to purchase a copy of the
photocopied
notes right away as we will be using some of them in class on Thursday.
Jason Criscio, one of the TAs, will hold office hours Monday from 3-5
pm in PAS 526. You can contact
him at criscio@atmo.arizona.edu. Christy King has not told me
what her office hours will be yet. The Physics and
Atmospheric Sciences (PAS) Building is located on 4th St. about half
way between Park and Highland Ave.
Next we looked at the Writing
Requirements
handout. You should be thinking about which of the experiments
(or book or scientific paper reports) you would like to do so that you
can sign up in class on Thursday. Distribution of the materials
for
the first experiment will probably begin next Tuesday (Aug. 29).
Your grade in this class will depend on your quiz scores, how much
extra credit you earn, your writing grade, and (perhaps) your
score on the final exam. A sample grade report from the Spring
2006 NATS 101 class was shown.

This student didn't have a high enough average to get out of
the final
exam (about 15-20% of the class will). Even though the
student had a low C average on the quizzes and earned a C on the
final
exam, extra credit and a high writing percentage grade
raised the student's overall average to a B.
We spent the next part of class listing the five most abundant gases in
the
earth's atmosphere. You can read about this and more in the first few
pages of the textbook (see the Reading
Assignments link on the class
web page).
What is the most abundant gas in the atmosphere? A few students
were pretty sure they knew the answer, several others were pretty sure
they did not. A clear cold liquid was poured from a thermos into
a styrofoam cup as a clue.

The liquid (and the most abundant gas in the atmosphere) was
nitrogen (you can fill in the blank above with the word
nitrogen). You can see liquid nitrogen. Once it has
evaporated and turned into a gas it is invisible.
Oxygen is the second most abundant gas in the atmosphere. Click here
to see a photograph of some liquid oxygen (it has a faint bluish color).
The remaining three gases on the list are shown below

Water
vapor and argon occupy 3rd and 4th place.
The variable concentration of water vapor means it is sometimes more
abundant & sometimes less abundant than argon.
As you read through the first part of Chapter 1 in the text you will
learn that the earth's original atmosphere was very different from
today's atmosphere. You will learn where our present atmosphere
came from and how it evolved over time (in particular how oxygen was
added to the atmosphere). More on this topic at the end of
today's notes.
You can see liquid water, just as was true with the liquid
nitrogen. Once water evaporates and forms water
vapor it is invisible. When you see steam, fog, or a cloud
you are seeing small drops of liquid water or small ice crystals not
water vapor.
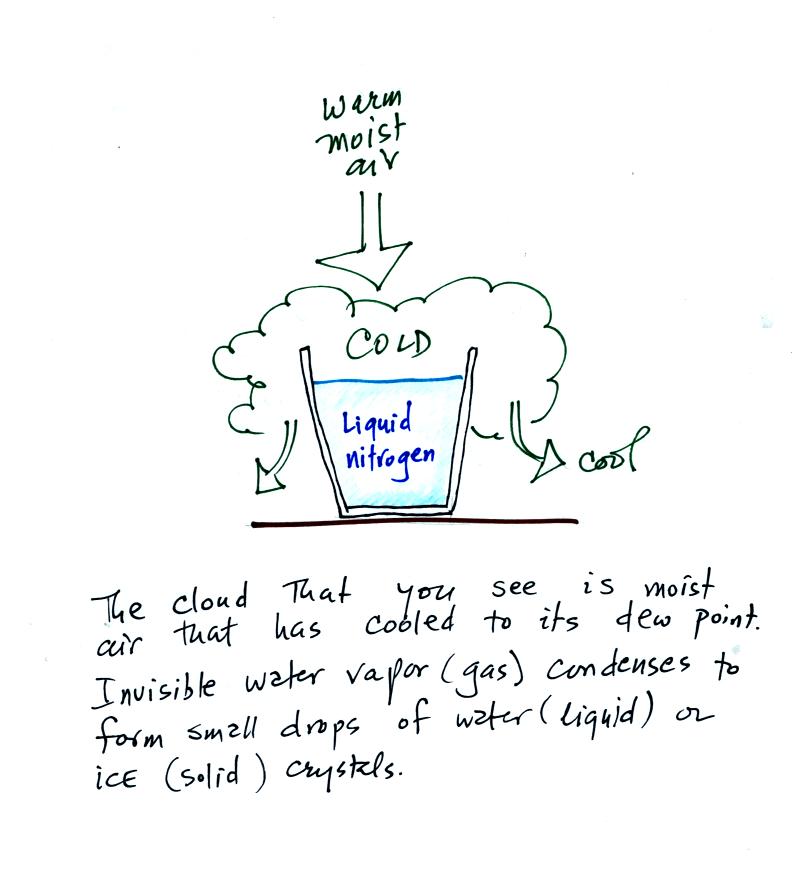
Water plays an important role in the formation of clouds, storms,
and weather. Meteorologists are very interested in knowing how
much water vapor is in the air at a particular time. They often
use the dew point temperature as a measure of water vapor amounts
rather than percentage concentration.
The figure below gives a rough equivalence between dew point
temperature and percentage concentration.

You can think of dew point as just being a number. When the value
is low the air doesn't contain much moisture. The higher the dew
point value, the more water vapor in the air.
We are currently in the middle of the summer thunderstorm season in
Arizona and dew points are in the upper 50s and lower 60s (the summer
monsoon begins officially in Tucson when the daily average dew point
temperature is 54o F or above for three days in a row.
Click here
to see current dew point temperatures across the U.S.
Here are
some other gases found in the earth's atmosphere that we
will cover. Most are found in very low concentrations but that
doesn't
mean they are not important.
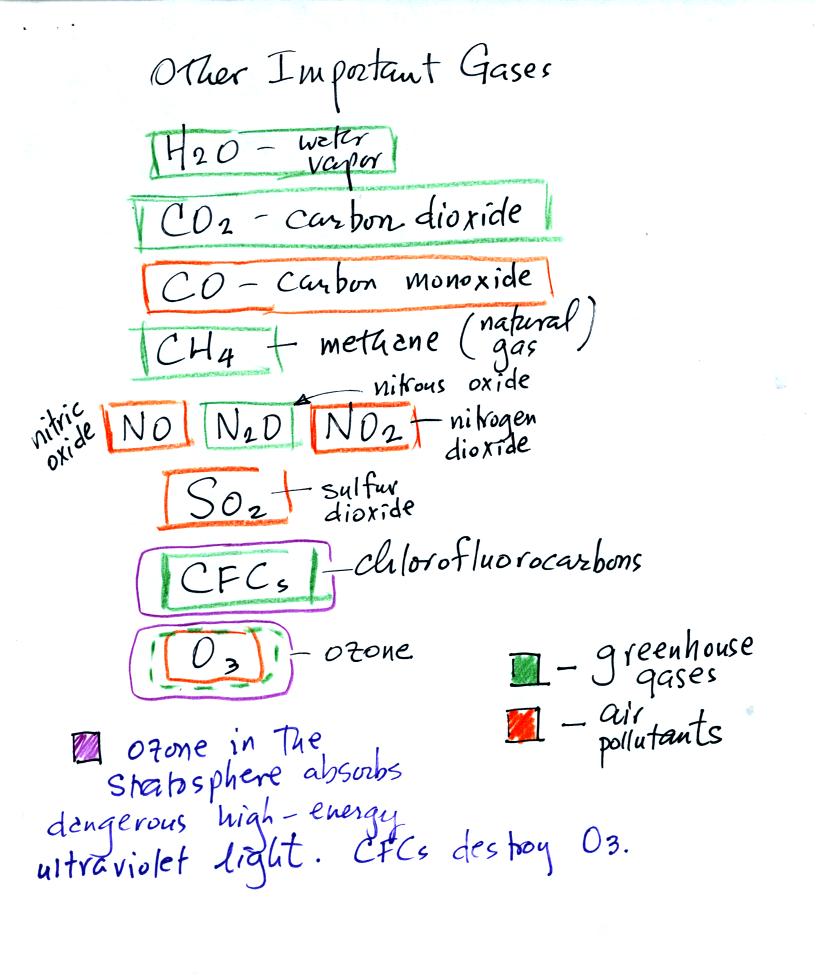
Water vapor, carbon dioxide, methane, nitrous oxide (N2O
=
laughing gas),
chlorofluorocarbons, and ozone are greenhouse gases. We will
cover the greenhouse effect in more detail when we get to Chapter
2. The "natural" greenhouse effect has a beneficial role on the
earth. Without the greenhouse effect average surface temperatures
on the earth would be much colder than they are now. Atmospheric
concentrations of many greenhouse gases are increasing however.
This could enhance or strengthen the greenhouse effect and cause global
warming which could have many detrimental effects.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the main air pollutants.
Ozone in the stratosphere absorbs dangerous high energy ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
Our
present atmosphere is composed mainly of nitrogen, oxygen, water vapor,
and argon. The earth's original atmosphere was very different.

The earth's first atmosphere was composed of hydrogen and helium.
These light weight gases escaped into space and were lost. The
next atmosphere was built up of gases emitted during volcanic
eruptions, mostly water vapor, carbon dioxide, and nitrogen. As
the earth began to cool the water vapor condensed and began to create
oceans. Carbon dioxide dissolved in the oceans and was slowly
turned into rock. Much of the nitrogen remained in the atmosphere.
Note the volcanoes didn't add oxygen to the atmosphere.
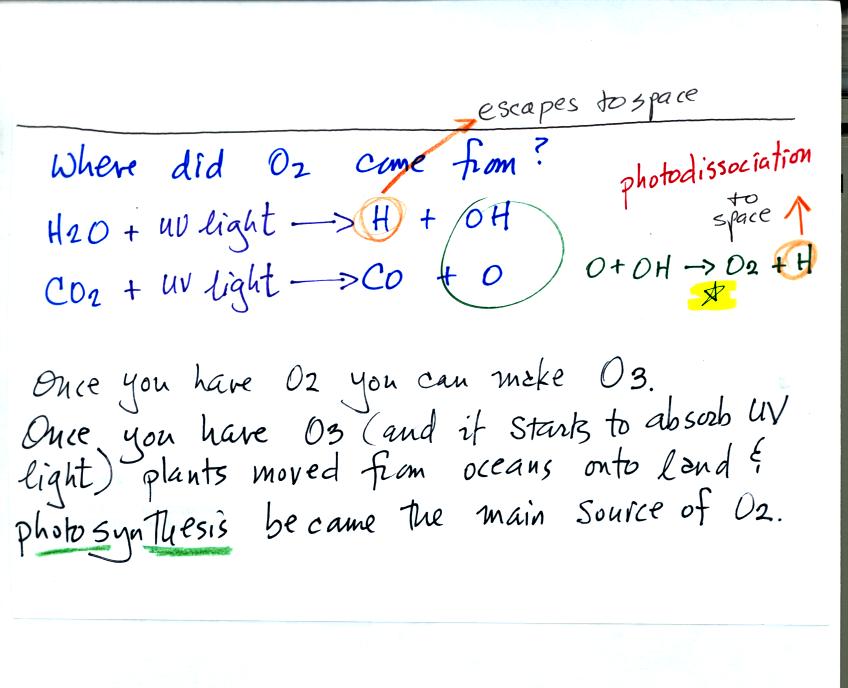
The oxygen is thought to have first come from photodissociation of
water vapor and carbon dioxide by ultraviolet light (the high energy
radiation splits the H20 and CO2 into
pieces). The O and OH react
to form O2 and H.
Once O2 begins to accumulate in the air it can react with O
to form
ozone, O3; The ozone then begins to absorb ultraviolet
light,
life forms can move from the oceans (which would absorb UV light in the
absence of ozone) onto land. Eventually plants and photosynthesis
would become the main source of atmospheric oxygen.







