Thursday Sept. 7, 2006
The first of the optional assignments
was handed out in class. It
will be due next Thursday (Sept. 14). You can earn a little bit
of extra credit on optional assignments. Optional assignments
should be ready to be turned in when you come to class. Don't let
the instructor see you finishing an optional assignment or working with
a friend in the last few minutes before the start of class (he is
likely to take your unfinished assignment and that of your friend).

Air pressure is a force that pushes downward, upward, and
sideways.
The bottom person in the people pyramid below must push upward with
enough
force to support the other people. The air
pressure in the four tires on your automobile push down on the road
(that's something you would feel if the car ran over your foot) and
push upward
with enough force to keep the 1000 or 2000 pound vehicle off the
road.


Three layers of air in the atmosphere are shown above (each
layer
contains the same amount of air, 10% of the air in the
atmosphere). This picture reminds you that air pressure decreases with
increasing altitude.
The layer at the ground and at the bottom of the
atmosphere is "squished" by the weight of the air above.
Squeezing all of this air into a thin layer or small volume increases
the air's density. The highest air density is found at the bottom
of the atmosphere.
The next layer up is also squished but not as much as the bottom
layer. The density of the air in the second layer is lower than
in the bottom layer. The air in the 3rd layer has even lower
density. It is fairly easy to understand that air density
decreases with increasing altitude.
Finally if you look closely at the figure you can see that pressure decreases most rapidly
with increasing altitude in the dense air at the bottom of the
atmosphere.
Next we'll
do a little demonstration (the demonstration doesn't involve dropping
water balloons as shown below).

All of the forces acting on the water balloon are shown in the next
figure.
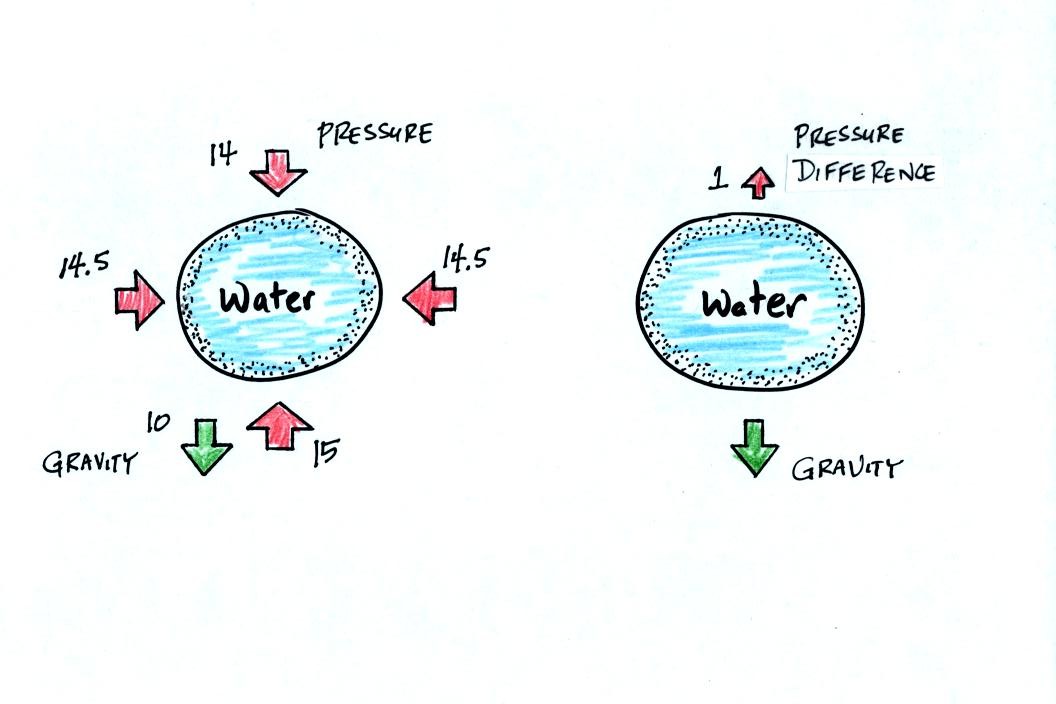
The figure at left shows air pressure (red arrows) pushing on all the
sides of the balloon. Because pressure decreases with increasing
altitude, the pressure pushing downward on the top of the balloon is a
little weaker than the pressure pushing upward at the bottom of the
balloon. The two sideways forces cancel each other out. The
total effect of the pressure is a weak upward force (shown on the right
figure, you might have heard this called a bouyant force).
Gravity exerts a downward force on the water
balloon. In the figure at right you can see that the gravity
force is stronger than the upward pressure difference force. The
balloon falls as a result.
In the demonstration a wine glass is filled with water. A small
plastic lid is used to cover the wine glass. You can then turn
the glass upside down without the water falling out.
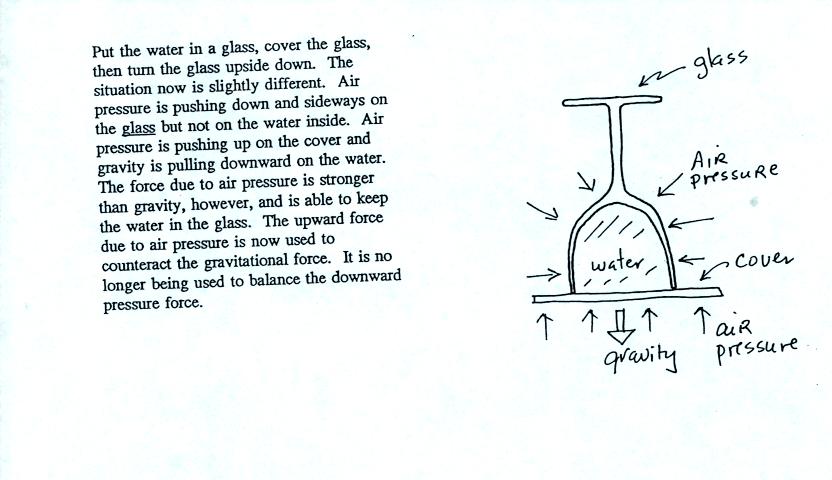
Now we'll look again at all of the forces and see how this is possible.

All the same forces are shown again in the left most figure. In
the right two figures we separate this into two parts. First
the water inside the glass isn't feeling the downward and sideways
pressure forces (because they're pushing on the glass). Gravity
still pulls downward on the water but the upward pressure force is able
to overcome the downward pull of gravity.
Some of
the historical information on pps. 31 & 32 in the photocopied class
notes was mentioned briefly.
Between 1926 and 1936 several courageous teams of scientists and
adventurers competed to see who could travel the highest in the
atmosphere by balloon. In May 1926 Captain Hawthorne Charles Grey
reached 28,510 ft. altitude. He was in an open gondola; note the
many layers of clothing that he needed in order to survive the cold
temperatures found at those altitudes.
Capt. Grey reached 42, 249 ft. At those heights, the air is too
thin to support life and supplementary oxygen is required. Grey
made another trip in November, 1926, but was killed when he ran out of
oxygen during the descent.
A short video tape segment documenting Auguste Piccard's and Paul
Kipfer's trip to 51,775 ft altitude was shown in class.

One of the test descents in the Trieste (the bathyscaph
designed and
built by Auguste Piccard) and a portion of Bertrand Piccard's
non-stop voyage around the globe by balloon will be shown in the next
week or two.
The Practice Quiz was administered in the final portion of the class.







