Tue., Sep. 12, 2006
"Storm Clouds" on the horizon (i.e. various things that are
due or coming due in the not too distant future)
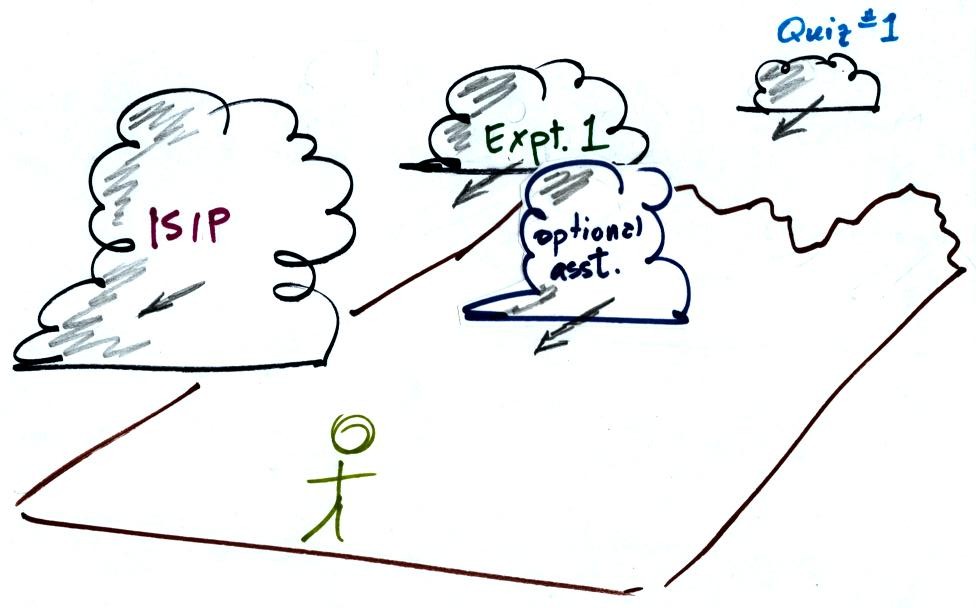
The 1S1P reports were
collected
today. It will take some time to grade all these
reports. You should expect to start seeing some of the reports
being returned next week.
The Optional Assignment is due Thursday (extra copies still available)
The Experiment #1 reports are due next Tuesday. You
should complete the experiment and return
your materials this week.
Quiz #1 is Thursday next week (Sept. 21). A preliminary
version of the Quiz #1 Study
Guide is now available.
There is a new reading assignment
Now don't get discouraged and think that there are only black clouds
headed our way. There are some good things coming this week
also. Here is one
of them, here is another.
The practice quizzes have been graded. Answers
to the questions are online. The average grade (60%) was quite a
bit lower than average grades in past NATS 101 courses. The
instructor made two suggestions: (1) that you get your notes organized,
keep everything (hand written notes and photocopied class notes)
together in one place and (2) that you briefly look over your notes
after each class (if they don't make sense then they won't make any
more sense in a week or two when it comes time to study for the next
quiz). If there is a part of one class that you don't understand
ask questions now, don't wait until just a day or two before the next
quiz.

Today we will try to understand why warm air rises and cold air
sinks. This will be a three step process.
When you fill a balloon with air you really aren't filling it with
air. You put some air molecules into the balloon but the inside
of the balloon is still mostly empty space. So what keeps the
balloon inflated? It is the rapid motions (they're moving around
at 100s of MPH) of the air atoms and molecules. When they collide
with and bounce off the inside walls of the balloon they push
outward. This outward force (divided by the area on which it is
exerted) is pressure. The ideal gas law equations tell you what
determines how strong or weak the pressure will be.
When you warm or cool a parcel (a volume) of air in the atmosphere, the
parcel will expand or shrink. It does this in an attempt to keep
the pressure inside the parcel constant. The pressure of the air
inside the parcel that is pushing out stays the same as the pressure of
the air outside the parcel pushing in. This is a special
situation involving the ideal gas law. This is called Charles'
Law. We will find that a parcel of air that is warmer than the
surrounding air will have lower density than the surrounding air.
Air that is colder than the surrounding air will have higher density
than the surrounding air.
Once we understand how air parcels behave when warmed or cooled we can
look at the forces that act on air parcels. Small changes in one
or the other of these forces will determine whether a parcel of air
rises or sinks (or just remains stationary).

The pressure produced by the air molecules inside a balloon will
first depend on how many air molecules are there.
As you add air to a bicycle tire the tire pressure increases.

Air pressure inside a balloon also depends on the size of the
balloon. Pressure is inversely proportional to volume, V
(increasing V decreases P and vice versa).
Note it is possible to keep pressure constant by changing N and V
together in just the right kind of way. This is what happens in
the experiment that some of you are working on. As oxygen is
removed from an air sample, the air sample volume decreases and
pressure of the air sample stays constant.

You shouldn't throw a can of spray paint into a fire. The
pressure of the gas inside a container depends on the gas temperature.

The volume of the can doesn't change. Heating the gas inside
the can increases the pressure. If the pressure gets high enough
the can explodes.

Surprisingly the pressure does not depend on the mass of the
molecules. Pressure doesn't depend on the composition of the
gas. Gas molecules with a lot of mass will move slowly, the less
massive molecules will move more quickly. They both will collide
with the walls of the container with the same force.
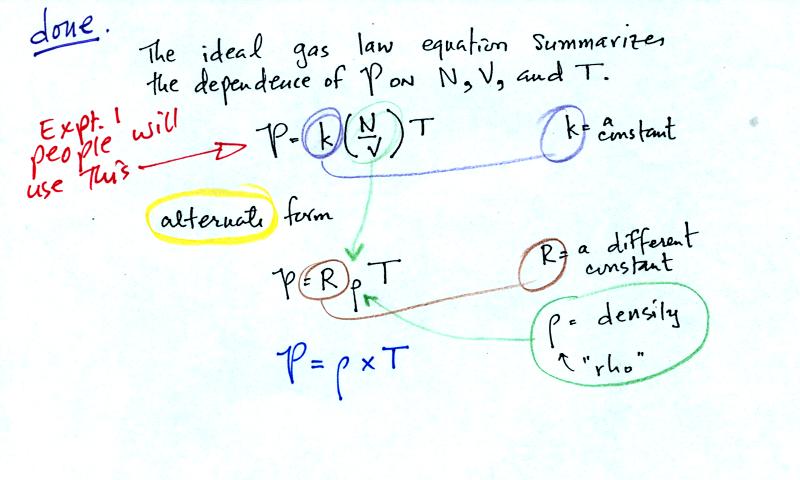
Here are the two ideal gas law equations. You can
ignore the
constants k and R if you are just trying to understand how a change in
one of the variables would affect the pressure. You only need the
constants when you are doing a calculation involving numbers.
(1) Pressure = (Number of air molecules) multiplied by temperature divided by volume
or
(2) Pressure = (density) multiplied
by (temperature)
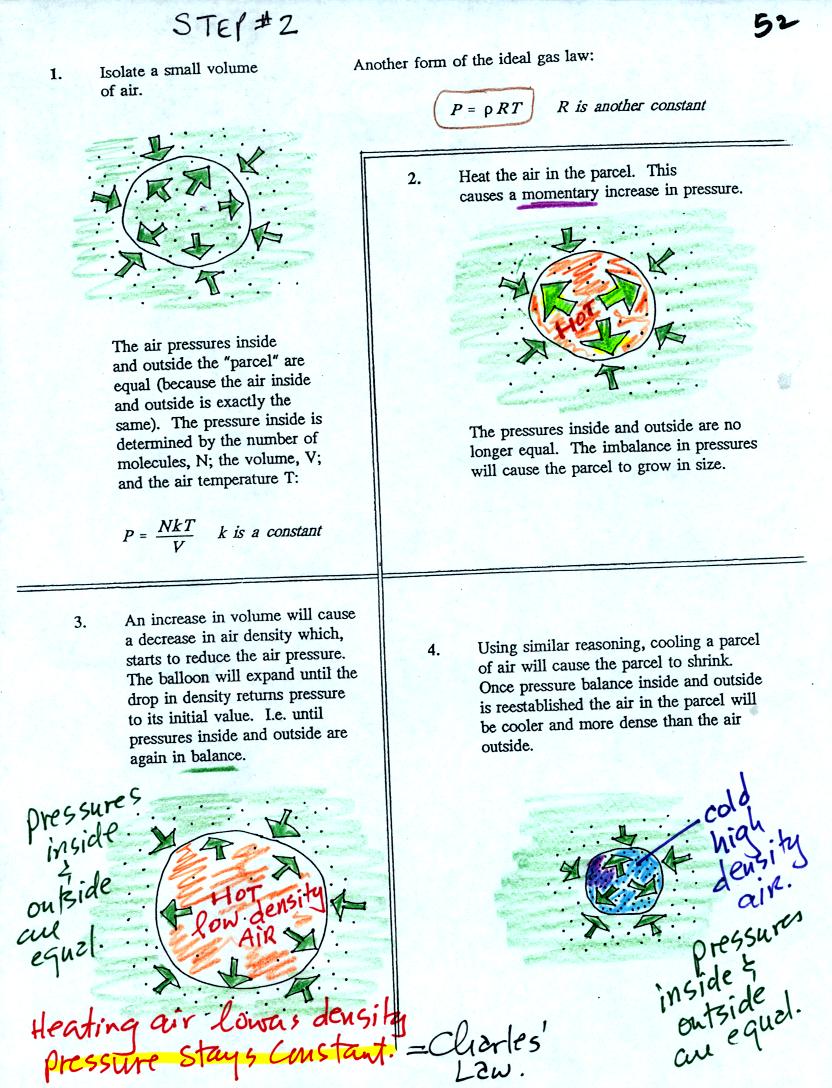
Air in the atmosphere behaves like air in a balloon. A
balloon can grow or shrink in size depending on the pressure of the air
inside.
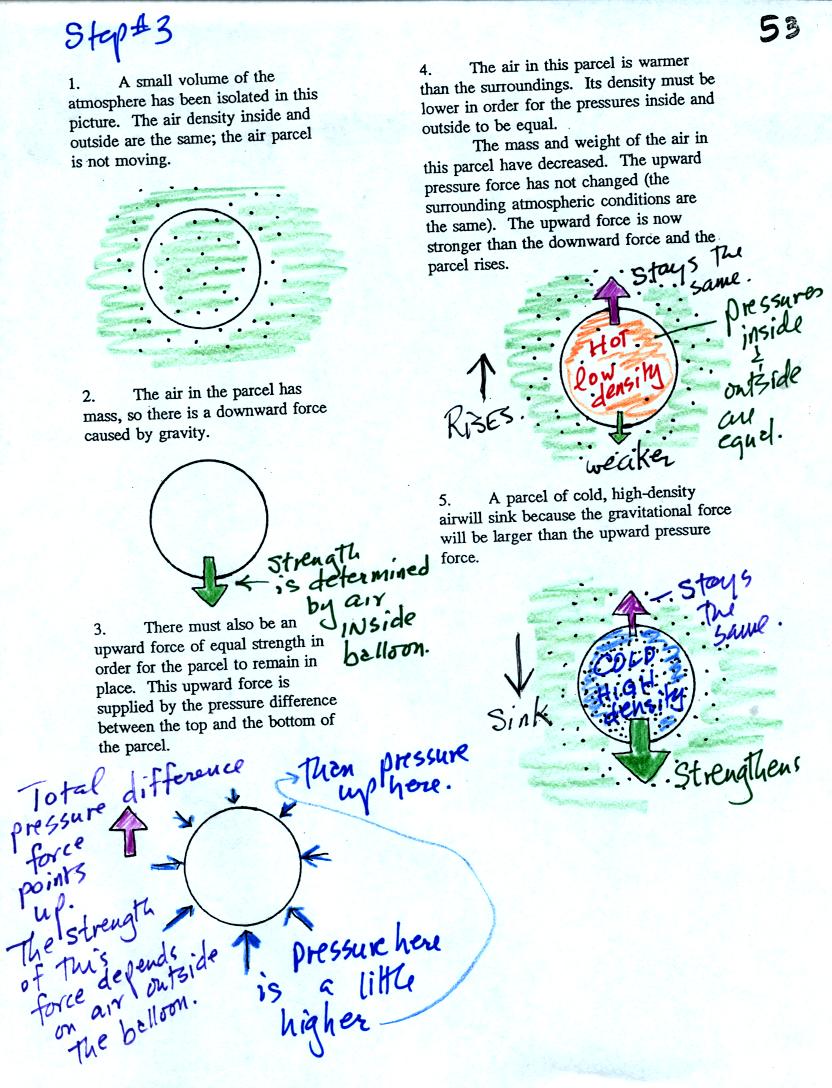
We start in the upper left hand corner with air inside a balloon that
is exactly the same as the air outside. The air inside and
outside have been colored green. The arrows show that the
pressure of the air inside pushing outward and the pressure of
the air surrounding the balloon pushing inward are all the same.
Next week warm the air in the balloon. The ideal gas law equation
tells us that the pressure of the air
in the balloon will increase. The increase is
momentary though. Because the pressure inside is now greater than
the pressure outside the balloon will expand. An increase in
volume will reduce the pressure of the air inside.
Eventually the balloon will expand just enough that the pressures
inside and
outside are again in balance. You end up with a balloon of warm
low density air that has the same pressure as the air surrounding it.
You can use the same reasoning to understand that cooling a balloon
will cause its volume to decrease. You will end up with a balloon
filled with cold high density air. The pressures inside and
outside the balloon will be the same.
These associations: warm air
= low density air and cold air = high density air are important and
will come up a lot during the remainder of the semester.
In the
atmosphere air temperature and air density change together in a way
that keeps pressure constant. This is Charles's Law and was
demonstrated in class. The demonstration is
illlustrated and described at the top of p. 54 in the photocopied
notes.

As temperature changes, the volume and density also change in a way
that keeps pressure inside the balloon constant (the pressure inside is
always staying equal to and in balance with the air pressure outside
the balloon).
Air in the atmosphere behaves like a balloon. A change in
temperature causes air density to change in
order to keep pressure inside and outside the balloon equal. We
will now look at the forces acting on a
parcel or balloon of air.
Air has mass and weight (Galileo may have been the first person to
prove that air had weight, here is a
description of the experiment that he may have
performed). When an air parcel has the same
temperature, pressure, and density as the air around it, the parcel
will remain stationary. With gravity pulling downward on the air,
there must be another force pointing upward of equal strength.
The upward force is caused by pressure differences between the bottom
(higher pressure pushing up) and top of the balloon (slightly lower
pressure pushing down on the balloon).
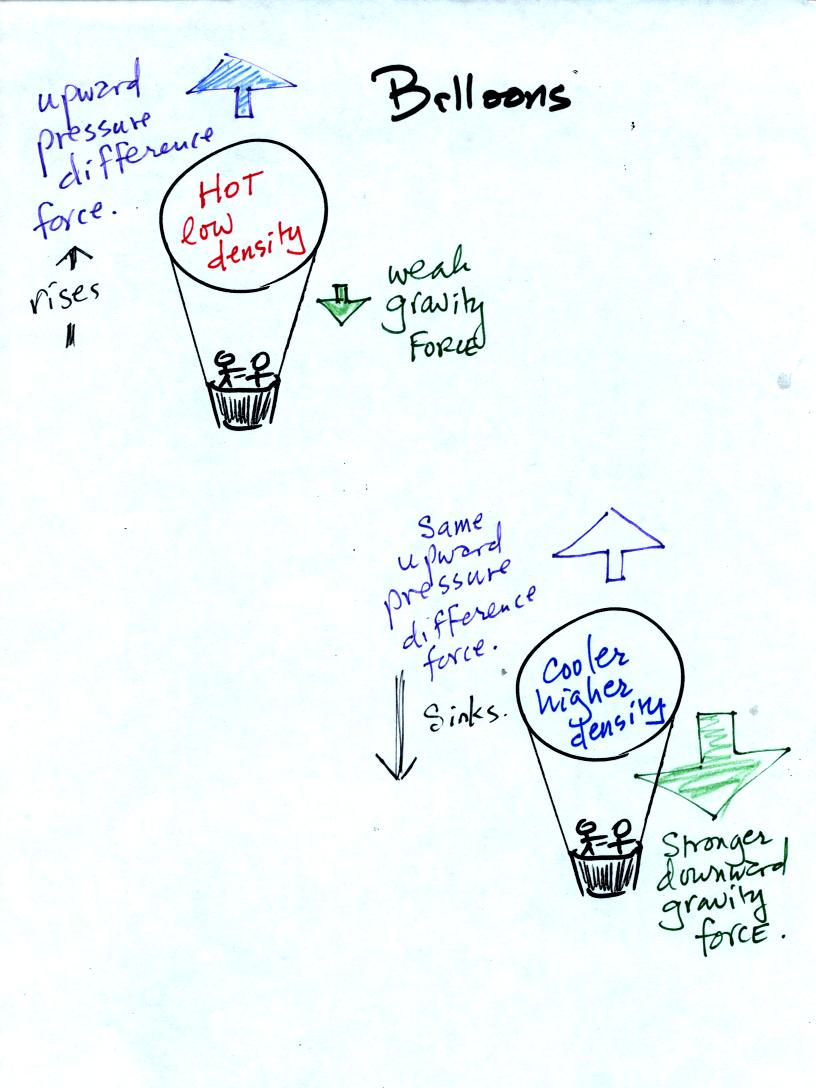
If the balloon is filled with warm, low density air the gravity force
will weaken (there is less air in the balloon so it weighs less). The
upward pressure difference force (which depends on the
surrounding air) will not change. The upward force will be
stronger than the downward force and the balloon will rise.
Conversely if a balloon is filled with cold low density air, gravity
will strengthen and the balloon will sink.
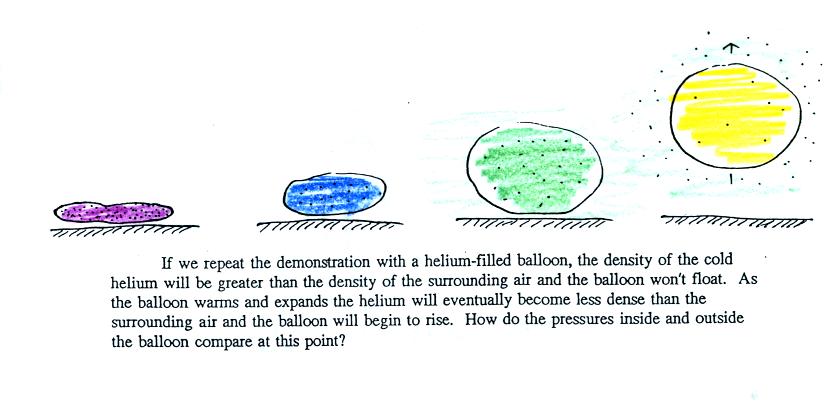
We modified the demonstration somewhat (see bottom of p. 54 in the
photocopied class notes). We used a balloon filled with helium
instead of air. Helium is less dense than air even when the
helium has the same temperature as the surrounding air. A helium
filled balloon doesn't need to warmed up in order to rise.
We dunked the helium filled balloon in some liquid nitrogen to cool it
and to cause the density of the helium to increase. When removed
from the liquid nitrogen the balloon can't rise, the gas inside is
denser than the surrounding air. As the balloon warms and expands
its density decreases. Eventually the balloon becomes less dense
than the surrounding air and lifts off from the table.
A balloon
pilot can adjust the temperature (and thereby the density) of the air
inside a balloon and make the balloon rise or sink.
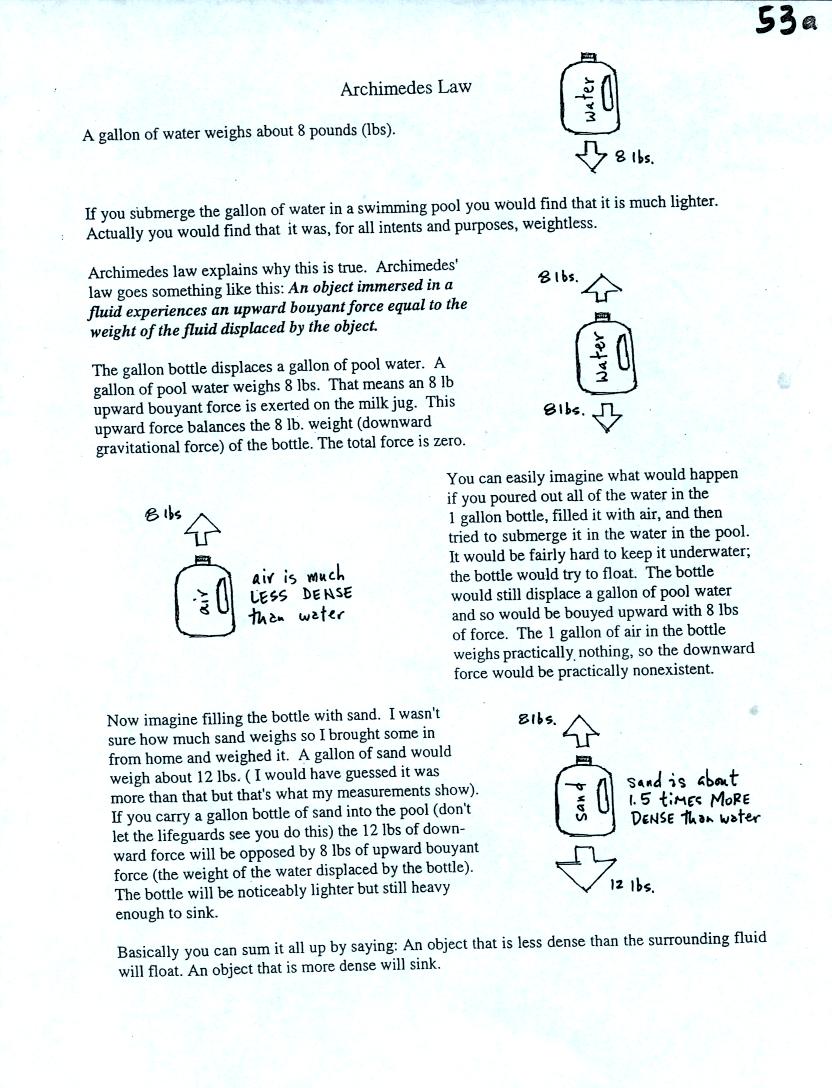
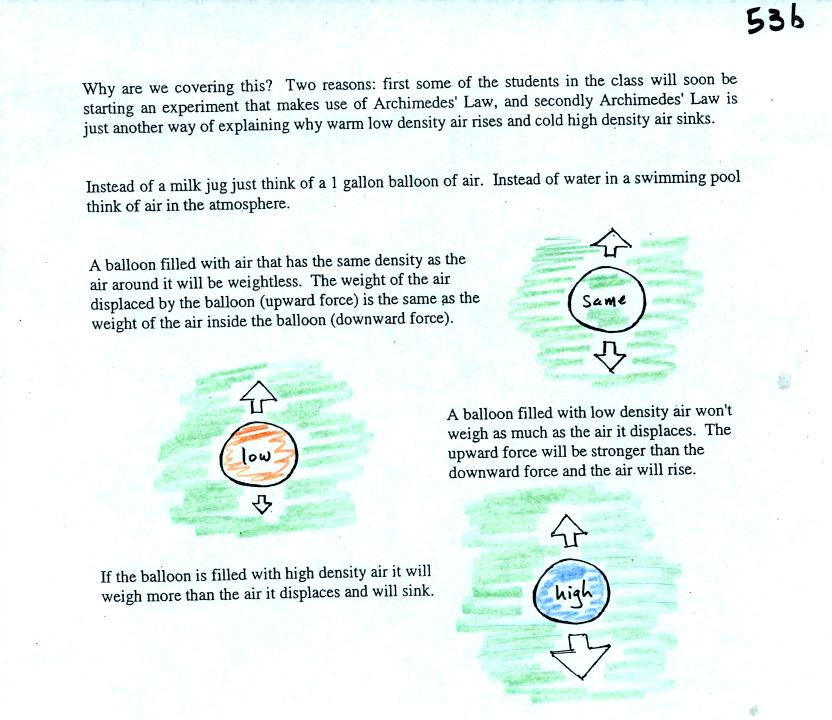
The upward
pressure difference force is really just the bouyant force in
Archimedes Law. Archimedes Law is another attempt to understand
and explain why objects float or sink. Archimedes Law is
discussed on pps 53a and 53b in the photocopied class notes. This
wasn't discussed in class. I did mention a hidden optional assignment, however.

In the last 10 or 15 minutes of class we watched a couple more video
segments. The first showed Auguste and Jacques Piccard's descent
to 10,000 ft. depth in the ocean in a bathyscaph. The second
discussed the first successful flight around the globe non-stop in a
balloon. Bertrand Piccard was a member of the two -man team.
















