
To cause condensation to occur you must cool the air to its dew
point temperature. Dew forms in the morning when the ground or
objects on the ground (like an automobile) cool and become colder than
the air above. When moist air comes into contact with the cold
ground, the air cools and condensation may occur. Your glasses or
sunglasses steam up when you leave a cold air conditioned room in the
summer and step outside into warm humid air. Water vapor
condenses when the moist air comes into contact with your cold
sunglasses. During condensation heat is released into the
surroundings.

Winds spiral into surface centers of low pressure. This is
convergence. Warm and cold fronts also cause air to rise.
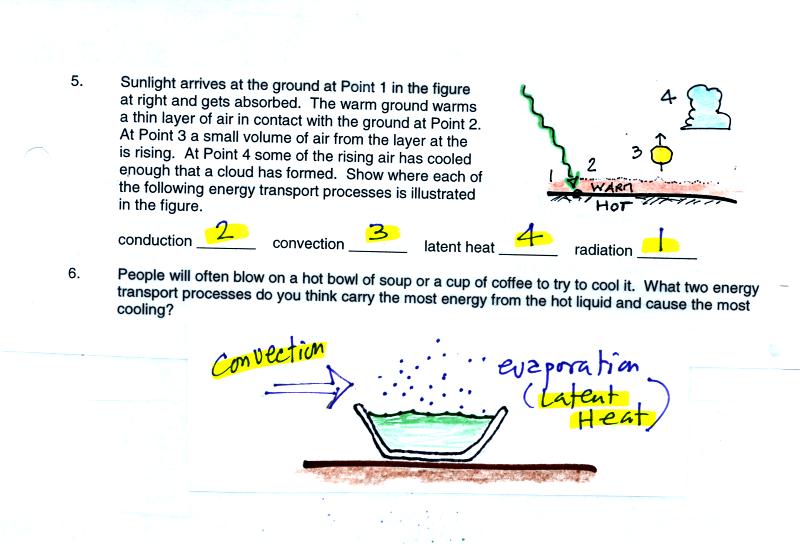
Blowing on a bowl of soup is convection. Hot soup will
evaporate. Evaporation takes energy from the soup and causes it
to cool. This would be latent het energy transport.
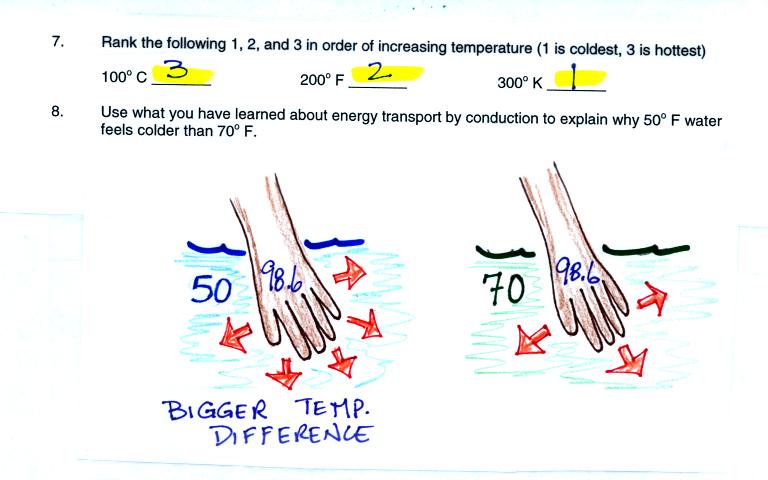
Our perception of cold is a measure of how much rapidly our body
is losing energy. Rate of energy loss by conduction depends on
the thermal conducitivity of the material and also on the temperature
gradient or temperature difference. There is a larger temperature
difference between your body and 50 F water than there is between your
body and 70 F water. The 50 F water will feel colder.
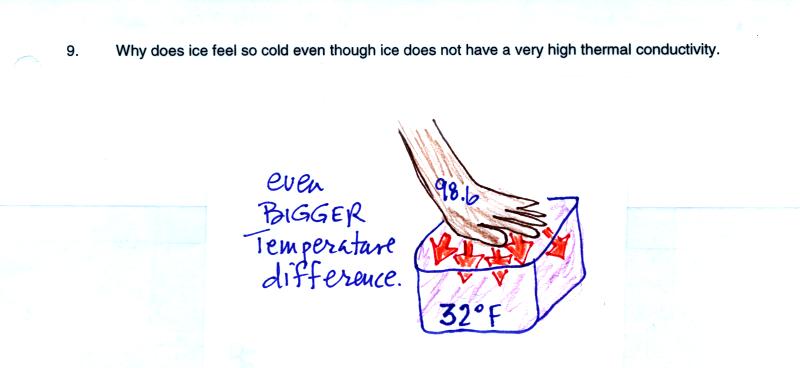
This is the same kind of situation as in Question 8. There is an
even larger temperature difference between your hand and a block of
ice. This is what causes the rapid loss of energy and the feeling
of cold.
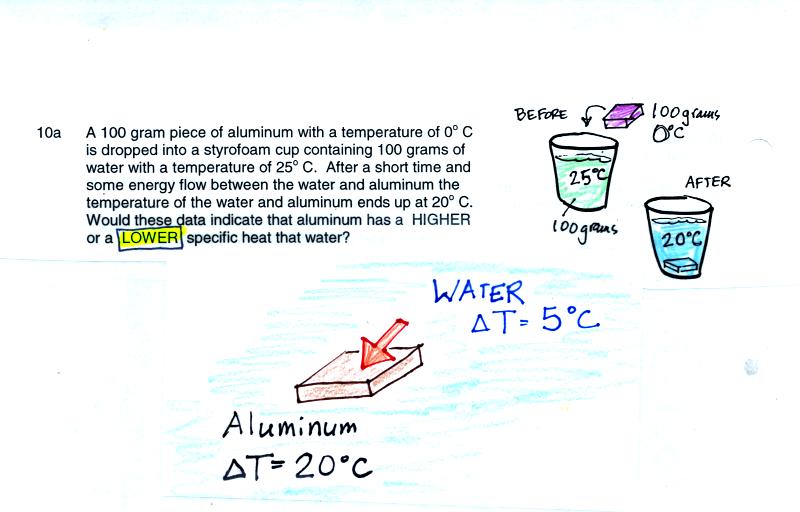
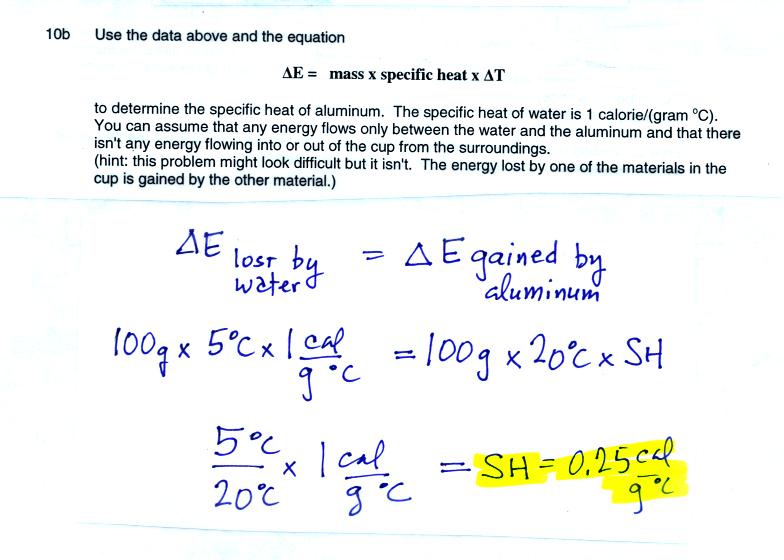
The first thing to realize in this question is the energy lost by the
water when it cools is the same energy gained by the aluminum as it
warms.
Then this is similar to a problem we did in class. In that case
we added equal amounts of energy to equal masses of water and soil and
calculated how much of a temperature change there would be for
each. The soil had a larger temperature change than the water
because the soil had lower specific heat.
Here the aluminum undergoes a larger temperature change which must be
because it has lower specific heat than the water.

Because water is involved you might conclude this was an example of
latent heat energy transport. But there is no mention of water
evaporating or water freezing, just organized motion of water.
This is therefore energy transport by convection.

In Question 13 the air on the right side is sinking because it has come
into contact with cold window glass and cooled. This increases
the density of the air and causes it to sinks. It warms and
begins to rise when it comes into contact with the other window pane.








