Monday
Aug. 20, 2007
The
first day of class.
We first briefly discussed the Course
Information
handout. Note the various options you have for purchasing a copy
of the course textbook. You should try to purchase a copy of the
photocopied
Classnotes (in the bookstore) right away as we will probably be using
some of
them in class on Wednesday.
Next we looked at the Writing
Requirements
handout. You should be thinking about which of the 4 experiments
(or book or scientific paper reports) you would like to do so that you
can sign up in class on Wednesday. Distribution of the materials
for
the first experiment could begin as early as Friday this week (Aug. 24).
Your grade
in this class will depend on your quiz scores, how much
extra credit you earn, your writing grade, and (perhaps) your
score on the final exam. A sample grade report from the Spring
2007 MWF Nats 101 class was shown.
Doe_J
quiz1 -36 (160 pts possible)
77.5%
quiz2 -46 (155 pts possible)
70.3%
quiz3 -48 (165 pts possible)
73.1%
quiz4 -35 (160 pts possible)
78.1%
2.6 EC points (3.3 pts possible)
writing scores: 33.0 (expt/book report) + 45.0
(1S1P pts)
writing grade: 97.5%
average (no quiz scores dropped): 79.3% + 2.6 =
81.9%
average (lowest quiz score dropped): 81.6% + 2.6 =
84.2
you DO need to take the final exam
-24.8 pts missed on the final exam = 75.3%
3Q&W>F overall average is 82.4
Don't
worry about all the details at this point. Note that this student
earned C grades on all the quizzes and the final exam but ended up with
a B in the class. This is due largely to the high writing grade
and the extra credit points
We'll
begin this new semester in Chapter 1 of the text. Before opening
the book and beginning the first reading
assignment, try to imagine
what you would put in the first chapter of a meteorology and
climatology textbook.

Student answers to the question above included: whether you
could breath the air, temperature of the outside air, nature of the
planet's surface, gravity, and pressure. Many of these are
covered in Chapter 1 of the text.
Today we
were concerned with the composition of the earth's atmosphere, in
particular the 5 most abundant gases in the earth's atmosphere.
Some of
the most abundant gas, in liquid form, was poured into a styrofoam cup.
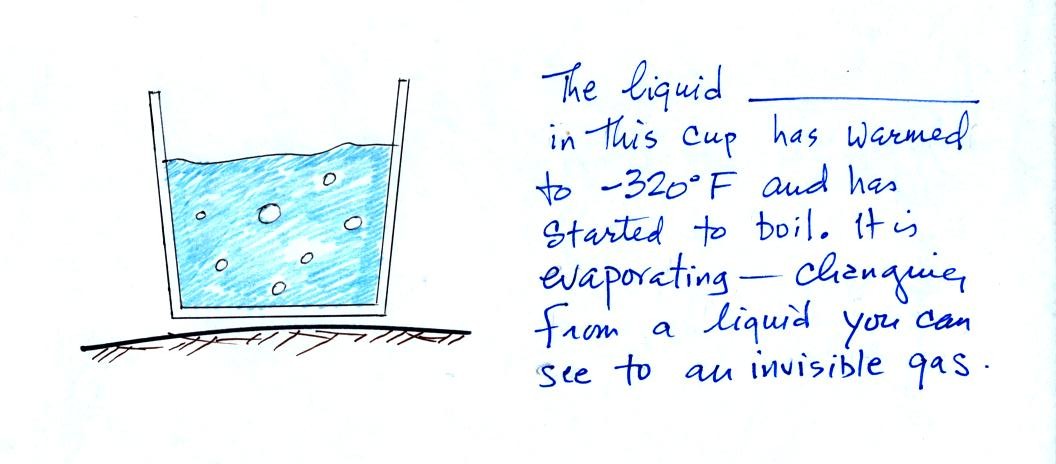
The liquid was
nitrogen (you can fill in the blank above with the word
nitrogen). You can see liquid nitrogen, it is clear and looks
like
water. Once it has
evaporated and turned into a gas it is invisible.
Oxygen is
the second most abundant gas in the atmosphere. Here's
a photograph of liquid oxygen. It has a light blue color.
Water vapor and argon are the 3rd and 4th most abundant gases in the
atmosphere. The concentration of water vapor can vary from near
0% to as high as 3% or 4%. The variable concentration
of water vapor means it is sometimes more
abundant, sometimes less abundant than argon which has a concentration
of about 1%.
Here's the complete list that we came up with in class:

Water plays an important role in the formation of clouds,
storms,
and weather. Meteorologists are very interested in knowing and
keeping track of how
much water vapor is in the air at a particular place and time.
One of the variables they use is the dew point temperature; it has two
"jobs."

Its first job is to provide
a measure of the amount of water
vapor in the air. The dew point is just a number. When
the value
is low the air doesn't contain much moisture. The higher the dew
point value, the more water vapor there is in the air.
The chart below gives a rough equivalence between dew point
temperature and percentage concentration of water vapor in the air.

We are in the summer thunderstorm season in Tucson and dew points
have been in the 60s. Many people use an evaporative cooler to cool their homes in
the summer. Evaporative coolers don't work very well when dew
points are as high as they are now.
Click here
to see current dew point temperatures across the U.S. Note that a
large part of the SE US currently has dew points in the 70s.
The second job of the dew point temperature is illustrated
below. When you cool moist air to its dew point, the
relative humidity becomes 100% and a cloud forms.

The following material wasn't covered
in class.
Our present atmosphere is very different from the earth's
original atmosphere.

The earth's first atmosphere was composed mainly of hydrogen
and
helium.
These light-weight gases escaped into space and were lost. The
next atmosphere was built up of gases emitted during volcanic
eruptions, mostly water vapor, carbon dioxide, and nitrogen. As
the earth began to cool the water vapor condensed and began to create
oceans. Carbon dioxide dissolved in the oceans and was slowly
turned into rock. Much of the nitrogen remained in the atmosphere.
Note the volcanoes didn't add oxygen to the atmosphere.
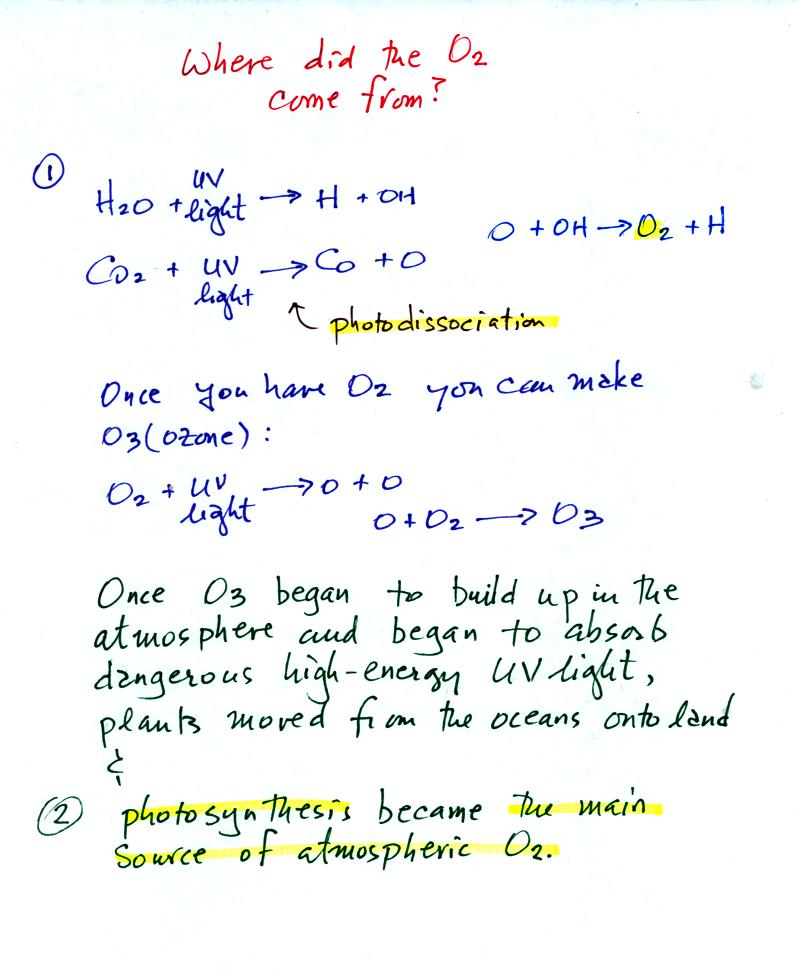
The oxygen is thought to have first come from
photodissociation of
water vapor and carbon dioxide by ultraviolet light (the high energy
radiation splits the H20 and CO2 into
pieces). The O and OH react
to form O2 and H.
Once O2 begins to accumulate in the air it can react with O
to form
ozone, O3. The ozone then begins to absorb ultraviolet
light,
life forms can move from the oceans (which would absorb UV light in the
absence of ozone) onto land. Eventually plants and photosynthesis
would become the main source of atmospheric oxygen.








