Wednesday Aug. 29, 2007
The Practice Quiz is one week from today. A preliminary version
of the Practice Quiz Study Guide is now
available online (there probably won't be many changes made between now
and next week). Note the location of the reviews held before the
practice quiz aren't yet known.
The collection of old quizzes from a previous semester of this course
are now available for purchase ($2.50).
You should expect to see the first 1S1P Assignment and the first
Optional (Homework) Assignment soon.
We'll
spend much of the period today covering stratospheric ozone.
But first there are a few things you should probably know about sulfur
dioxide.

Sulfur dioxide is produced by the combustion of sulfur
containing
fuels such as coal. Combustion of fuel also produces carbon
dioxide and carbon monoxide. People probably first became aware
of sulfur dioxide because it has an unpleasant smell. Carbon
dioxide and carbon monoxide are odorless.
Volcanoes are a natural source of sulfur dioxide.

The Great London smog is still the deadliest air pollution
event in
history. Because the atmosphere was stable, SO2
emitted into air
at ground level couldn't mix with cleaner air above. The SO2
concentration was able to build to dangerous levels. 4000 people
died during this 4 or 5 day period. The sulfur dioxide didn't
kill them directly. They had a prexising condition of some
kind. The SO aggravated their condition and contributed to their
death.
London type smog which contains sulfur dioxide and is most common
during the winter is very different from photochemical or Los Angeles
type smog. Los Angeles type smog contains ozone and is most
common in the summer.
Some other air pollution disaster also involved high SO2
concentrations. The Donora Pennsylvania event is described on p.
346 in the textbook.
We'll come back to sulfur dioxide briefly in class on Friday.

Stratospheric ozone forms naturally when UV light splits
oxygen
molecules (O2) into two oxygen atoms
(photodissociation). The O atoms can
then react
with unsplit O2 to make O3 ozone. The figure
above and the figure below are found on p. 17 in the photocopied
classnotes.
One way in which is destroyed naturally are shown in the figure
above. The ozone molecule is destroyed when it absorbs UV
light. The reactions in brackets shown how ozone can be destroyed
by reacting with atomic oxygen or with another ozone molecule.
Once you understand how stratospheric ozone is formed you can
appreciate why the peak concentrations (the ozone layer) are found not
at the bottom or
top of the atmosphere but at some level in between (at around 25 km),
where there are optimal amounts of oxygen and UV
light.
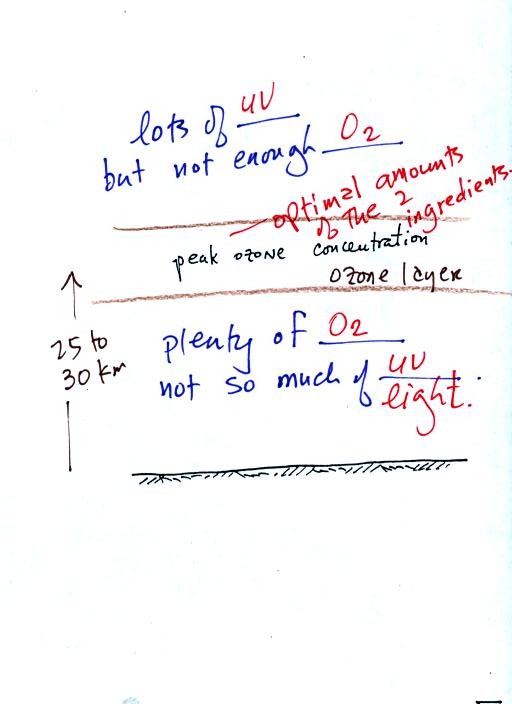
There is lots of UV light above 25 km but not much
oxygen. There
is plenty of oxygen below 25 km but not enough UV light. The
optimum amounts of both ingredients are found near 25 km.

Stratospheric ozone, the ozone layer, absorbs much (but not
all) of the dangerous high energy ultraviolet light from the sun.
Listed above are some of the serious hazards or problems associated
with exposure to ultraviolet light.

Human activities add substances to the atmosphere that can
potentially
reduce ozone concentration in the ozone layer (which would result in
increased exposure to UV light at the ground).
The first set of reactions above involve nitric oxide, NO. First,
NO reacts with O3 to form NO2 and O2 (ordinary molecular oxygen).
Then notice the NO2 reacts with an oxygen atom (which might otherwise
react with O2 to make O3) to form NO again and O2. The NO is available
again to react with and destroy another ozone molecule.
At one time many countries were considering building fleets of
supersonic aircraft that would fly into the stratosphere. The
plans were scrapped partly due to concern that the NO emissions from
these aircraft would damage the ozone layer.
The main threat now comes from chlorofluorocarbons (CFCs). The
reactions involving CFCs have been copied onto the next figure.
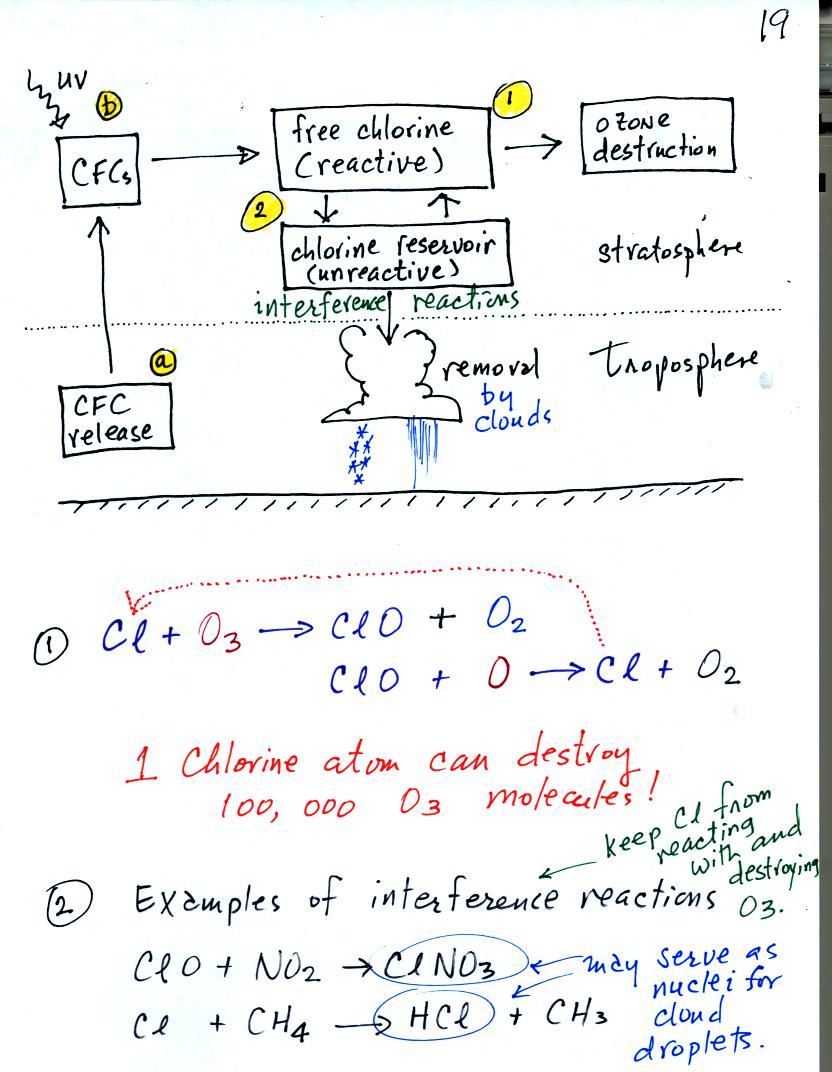
CFCs were at one time thought to be an ideal industrial chemical.
CFCs are unreactive, non toxic, and stable. Once they get into
the atmosphere they remain there a long time, as much as 100 years.
CFCs released at ground level [point (a) in the figure above]
remain in the atmosphere long enough that they can eventually make
their way up into the stratophere. UV light can break chlorine
atoms off the CFC molecule [ Figure (b) above]. The resulting
"free chlorine" can react with and destroy ozone. This is shown
in (1) above. Note how the chlorine atoms reappears at the end of
the two step reaction. A single chlorine atom can destroy 100,000
ozone molecules.
There are ways of removing chlorine from the atmosphere. A couple
of these so called "interference reactions" are shown in (2)
above. The reaction products might serve as
condensation nuclei for cloud droplets (the small water drops that
clouds are composed of) or these gaseous products might dissolve in the
water in clouds. In either event the chlorine containing chemical
is removed from the atmosphere by falling precipitation. Clouds
are probably the most effective way of cleaning the atmosphere.
The following information about the ozone
hole was not covered in class.
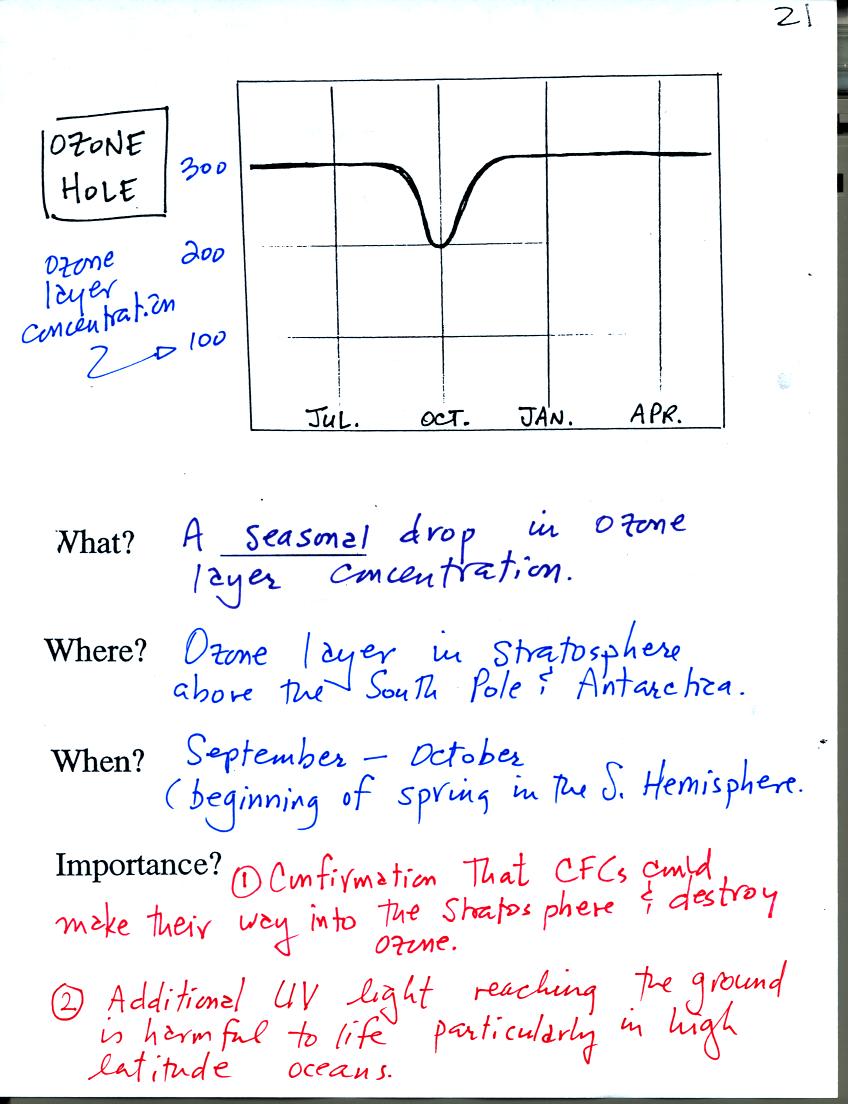
The ozone hole that forms above the S. Pole every year
around October
was one of the first real indications that CFCs could react with
and destroy stratospheric ozone. The hole is not really a hole in
the ozone layer, rather the ozone layer thins (concentration drops)
significantly.

The discussion above explains how extremely cold
temperatures and an
unusual wind pattern above the S. Pole in the winter are thought to
create the ozone hole when the sun returns in the spring.
Basically a new series of reactions (that take place on the surface of
cloud particles) interfere with the interference reactions. The
interference reactions would ordinarily keep chlorine from reacting
with and
destroying ozone. Interfering with those interference reactions
makes the chlorine available again to react with and destroy
ozone.
Chlorine containing compounds build up during the winter and are able
to destroy ozone once the sun returns in the spring.
The middle
portion of Chapter 1 looks at how atmospheric characteristics such as
air temperature, air pressure, and air density change with
altitude. In the case of air pressure we will spend some time
trying to understand what pressure is and what can cause it to change.
We will start by looking at how air temperature changes with altitude
because that is a property that were are able to feel and are probably
most familiar with.
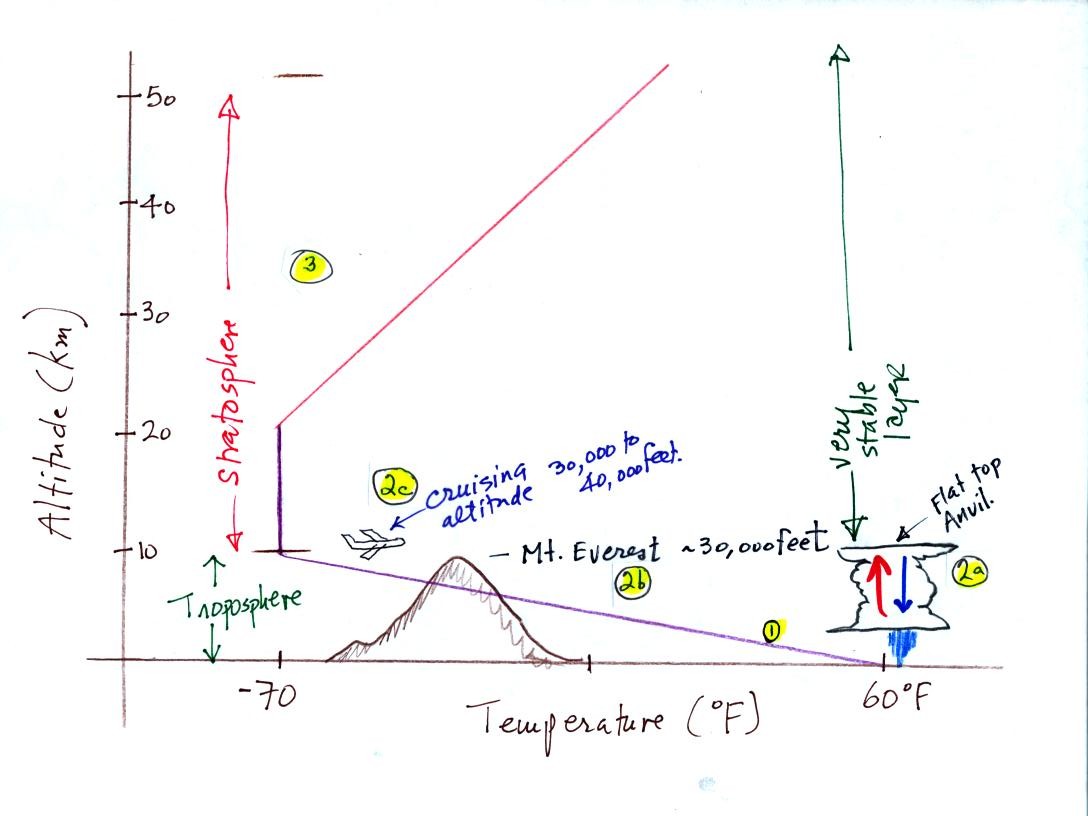
Here is a picture we started but didn't quite finish in class. (the
highlighted numbers
1 - 3 were added after class
to aid with the discussion of this
figure)
The atmosphere can be split
into layers
depending on whether
temperature is increasing or decreasing with increasing altitude.
The two lowest layers are shown in the figure above. There are
additional layers (the mesosphere and the thermosphere) above 50 km but
we won't worry about them.
1. We live in
the troposphere. The troposphere is found, on average, between 0
and about 10 km altitude, and is where temperature usually decreases
with
increasing altitude.
The troposphere contains most of the water vapor
in the atmosphere and is where most of the weather occurs. The
troposphere can be stable or unstable (tropo means to turn over and
refers to the fact that air can move up and down in the
troposphere).
2a. The thunderstorm shown in
the figure indicates unstable conditions, meaning that strong up and
down air motions are occurring. When the thunderstorm reaches the
top of the troposphere, it runs into the stable stratosphere. The
air can't continue to rise into the stable stratosphere so the cloud
flattens out and forms an anvil (anvil is the name given to the flat
top of the thunderstorm). The flat anvil top is something
that you can see and often marks the top of the troposphere.
2b. The summit of Mt. Everest is nearly 30.000 ft. tall and is
close to the top of the troposphere.
2c. Cruising altitude in a passenger jet is usually between
30,000 and 40,000, near or just above the top of the troposphere.
3. Temperature remains constant between 10 and 20 km
and then
increases with increasing altitude between 20 and 50 km. These
two sections form the stratosphere. The stratosphere is a
very stable air layer. Increasing temperature with increasing
altitude is called an
inversion. This is what makes the stratosphere so stable.
The figure was redrawn and some
additional information was added after
class.
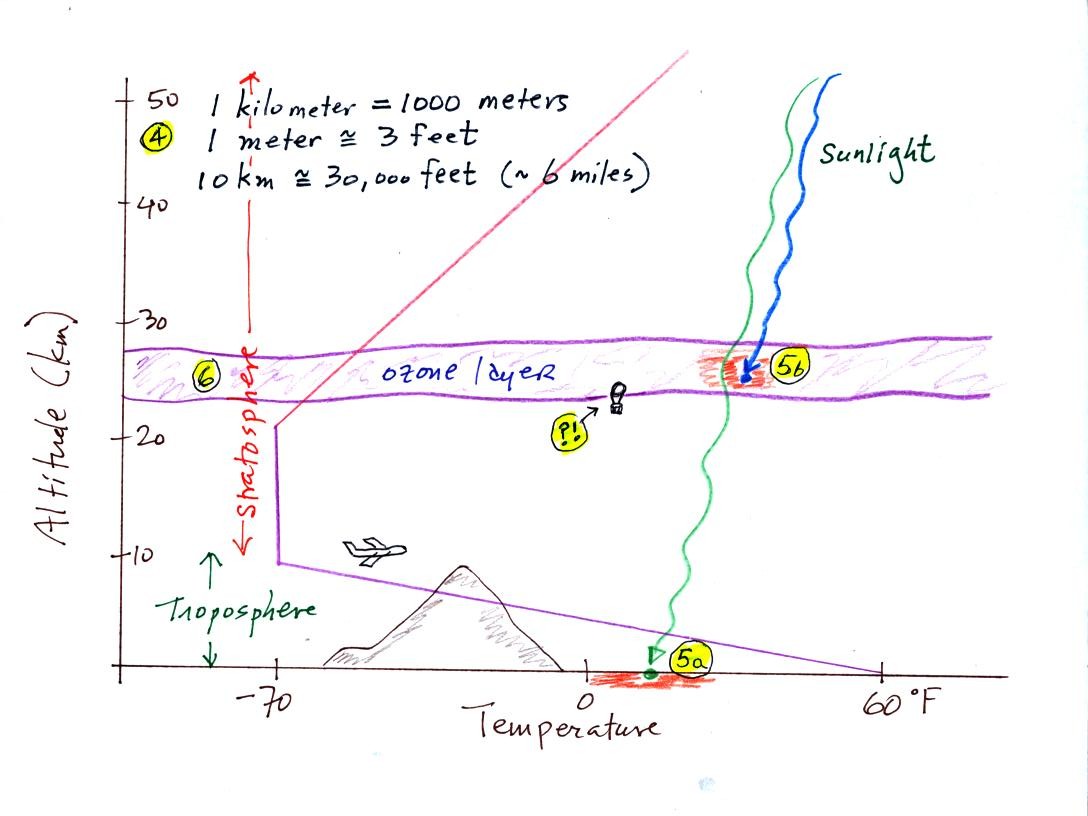
4. 10 km (kilometers) is approximately 30,000 feet or
about 6 miles.
5a. Much of the sunlight arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground. This warms the ground. The air in contact with the
ground is warmer than air just above. As you get further and
further from the warm ground, the air is colder and colder. This
explains why air temperature decreases with increasing altitude.
5b. How do you explain increasing temperature with
increasing
altitude in the stratosphere.
Absorption of
ultraviolet light by ozone warms the air in the stratosphere and
explains why the air can warm. The air in the stratosphere is
much less dense (thinner) than in the troposphere. It doesn't
take as much energy to warm this thin air as it would to warm denser
air closer to the ground.
6. The ozone layer is found in the
stratosphere (peak concentrations are found near 25 km altitude).
Point ?!
That's a manned balloon, Auguste Piccard and Paul Kipfer are
inside. They were to first men to travel into the
stratosphere (see pps 31 & 32 in
the photocopied Class Notes). We'll see a short video showing
part of their adventure in the next week or so.










