Friday Sept. 21, 2007
Quiz #1 was returned in class. Please check your quiz carefully
for grading errors and make sure the points were added up correctly.
There is a new Chapter 2 reading
assignment.
Chapter 2
is concerned with energy, temperature, heat, energy transport, energy
balance between the earth, atmosphere, and space. It is easy to
lose sight of the main concepts because there are so many
details. The following (found on pps 43&44 in the photocopied
Class Notes) is meant to introduce some of what we will be covering in
class from Chapter 2.

We will learn the names of several different types or forms of
energy. Kinetic energy is energy of motion. Some examples are mentioned
and sketched above. It is a relatively easy to visualize and
understand form of energy.
Latent heat energy is perhaps the most underappreciated and most
confusing type of energy. The word latent refers to energy that is
hidden in water and water vapor. This energy can emerge when
water vapor condenses or water freezes.
Radiant energy is a very important form of energy that was for some
reason left off the original list. Sunlight is an example of
radiant energy that we can see and feel (you feel warm when you stand
in and absorb sunlight). There are many types of radiant energy
that are invisible.
The four energy transport
processes are listed below.

By far the
most important process is electromagnetic radiation (light is a common
form of electromagnetic radiation). This is the
only process that can transport energy through empty space.
Electromagnetic radiation is also responsible for about 80% of the
energy transport between the ground and atmosphere.
You might be
surprised to learn that latent heat is the second most important
transport process.
Rising parcels of warm air and sinking parcels of cold air are
examples of convection. Because of convection you feel colder or
a cold windy day than on a cold calm day. Note that convection is
a 3rd way of causing rising air motions in the atmosphere (convergence
into centers of low pressure, and fronts were the other two ways).
Ocean currents are also an example of convection. Ocean currents
transport energy from the warm tropics to colder polar regions.
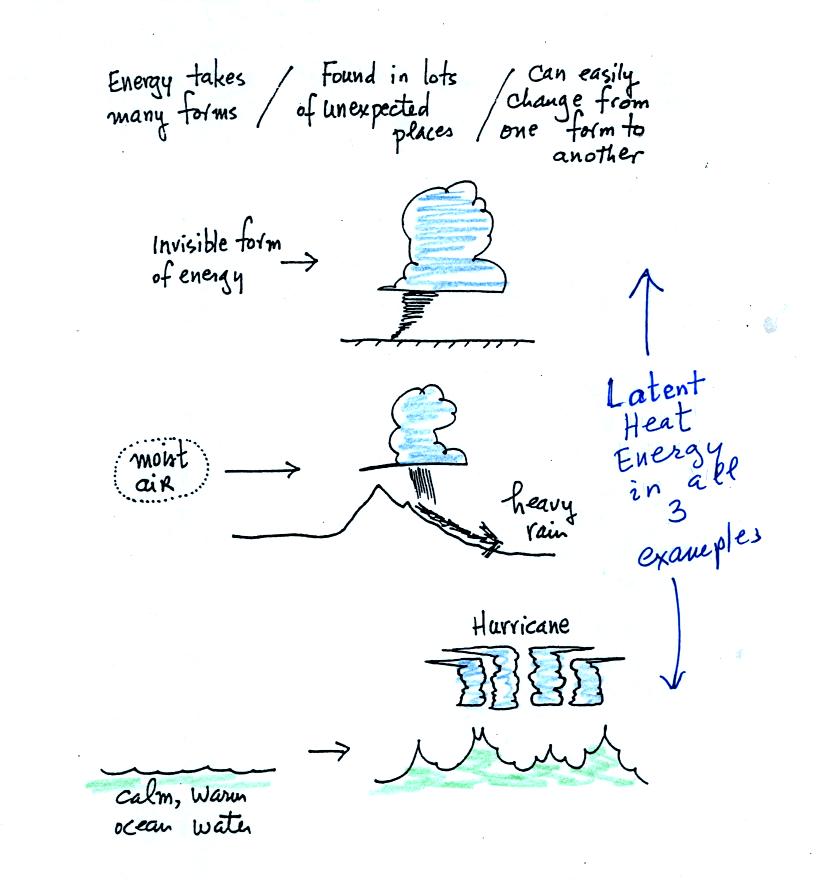
Water vapor is a particularly important form of invisible
energy.
When water vapor condenses to produce the water droplets (or ice
crystals) in a
cloud, an enormous amount of latent heat energy is released into the
atmosphere.
It is hard to visualize or appreciate the amount of energy released
into the
atmosphere during condensation. You can imagine the work that you
would do carrying a gallon of water
(8 pounds) from Tucson to the top of Mt. Lemmon. To
accomplish
the same thing Mother Nature must first evaporate the water and (if my
calculations are correct) that requires about 100 times the energy that
you would use to carry the 8 pounds of water to the summit of Mt.
Lemmon. And Mother Nature transports a lot more than just a
single gallon.
The next picture shows energy being transported from the sun to
the earth in the form of electromagnetic radiation.
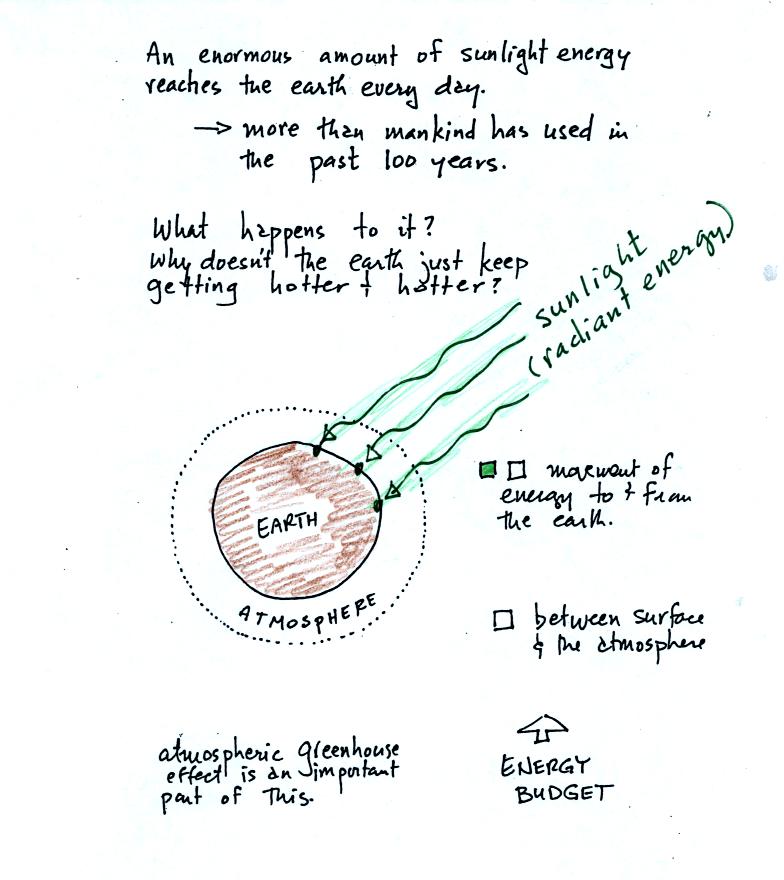
We are aware of this energy because we can see it (sunlight
also contains invisible forms of light) and feel it. With all of
this energy arriving at and
being
absorbed by the earth, what keeps the earth from getting hotter and
hotter? The answer is that the earth also sends energy back into
space (the orange and pink arrows in the figure below)
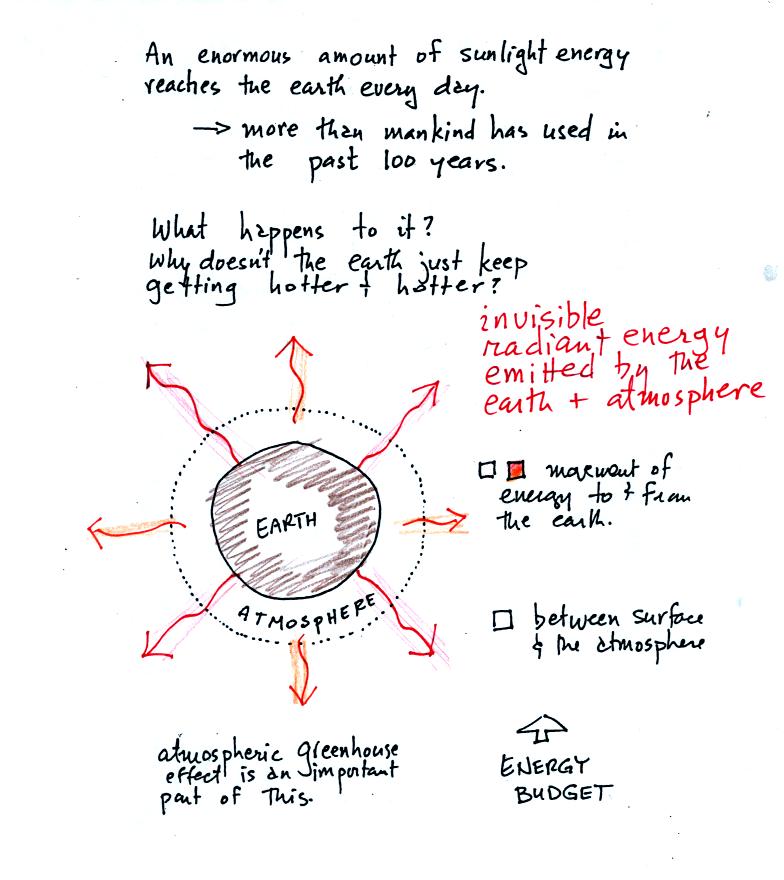
This infrared light is an
invisible form of energy (it is weak enough that we
don't usually feel it either). A balance
between incoming and outgoing energy is achieved and the earth's annual
average temperature remains constant.
We will also look closely at energy transport between the earth's
surface and the atmosphere. This is where latent heat energy transport
and convection and conduction operate (they can't transport energy past
the atmosphere and into outer space).
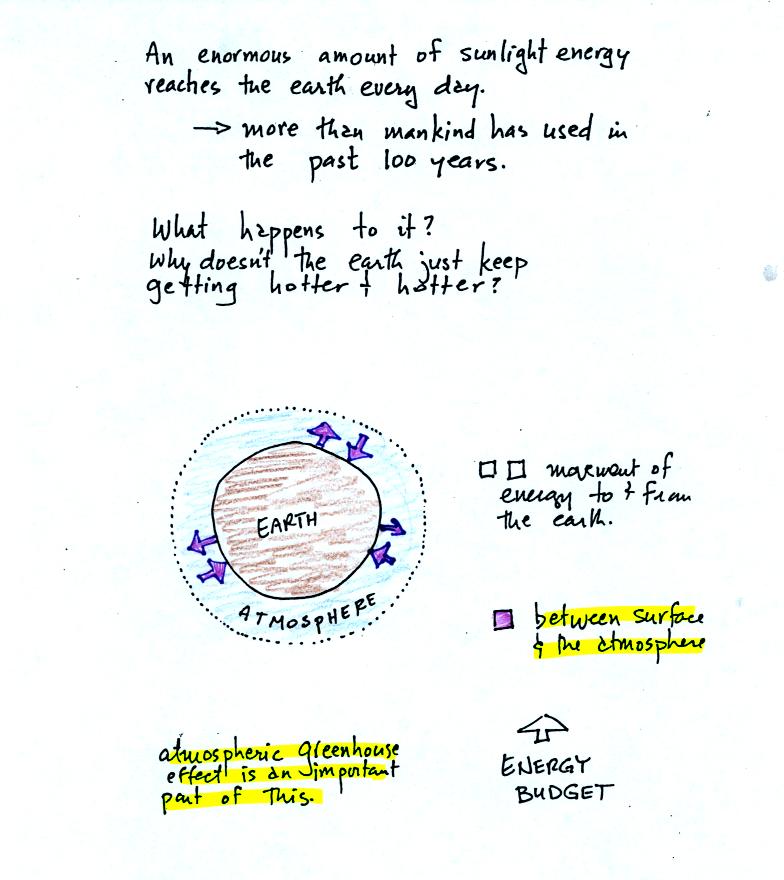
That is also where the atmospheric
greenhouse operates. That will be a important goal in Chapter 2 -
to
better understand how the atmospheric greenhouse effect works.
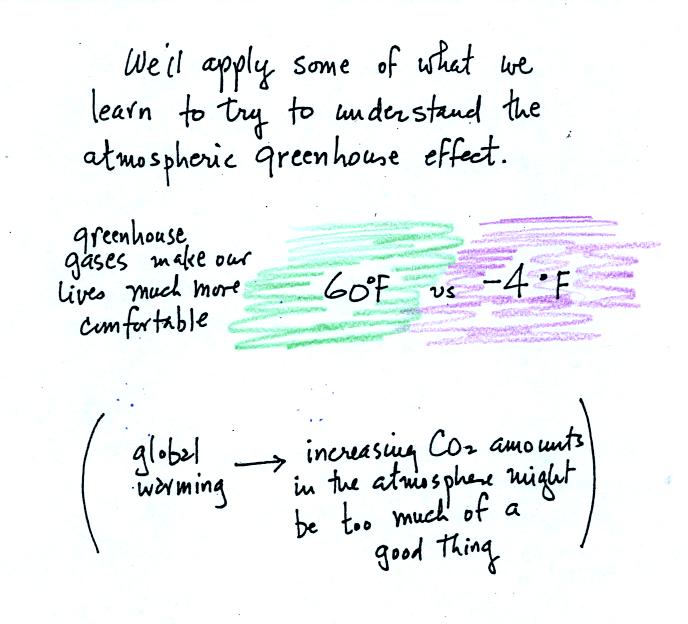
Remember that without the greenhouse effect, the global annual
average surface temperature on the earth would be about 0o F
rather
than 60o F.
I've taken
the information on p. 45 in the photocopied notes and split it into two
parts.
When you add energy to an object, the object will usually
warm
up. It is relatively easy to come up with an equation that allows
you to figure out what the temperature change will be.
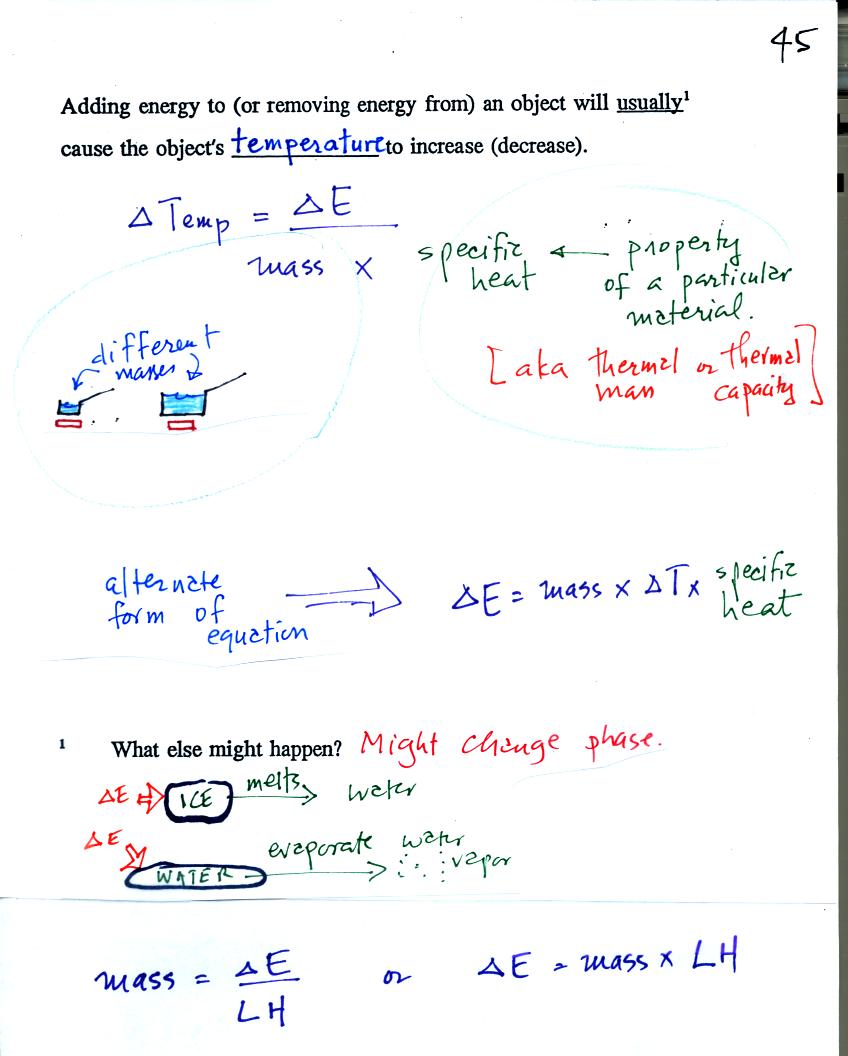
When you add energy to something the temperature change will
depend on
how much energy was added. So delta E is in the numerator of the
equation. When you add equal amounts of energy to a small pan of
water and to a large pan of water, the small pan will heat up more
quickly. The temperature change, delta T, will depend on the
mass. A large mass will mean a small delta T, so mass should go
in the denominator of the equation. Different materials
react differently when energy is added to them. A material with a
large specific heat will warm more slowly than a material with a small
specific heat. Specific heat behaves in the same kind of way as
mass. Specific heat is sometimes called "thermal mass."
Note the alternate form of the equation. If you know the mass and
specific heat of an object and observe the object warm or cool, you
could use a measurement of the temperature change (delta T) to
calculate how much energy was
added to or removed from the object. We made use of this form of
the equation in a class experiment.
An object will usually warm when you add energy to it. But there
is another possibility (mentioned at the bottom of the figure).
The object could change phase (change from solid to liquid or
gas). Adding energy to ice might cause
the
ice to melt. Adding energy to liquid nitrogen could cause the
nitrogen to
evaporate and turn into nitrogen gas.
The two equations at the bottom of the figure above allow you predict
how much of a material will melt
(or evaporate) if a certain amount of energy is added to it. Or
if you want to melt (evaporate) a certain amount of material the second
equation could be used to compute how much energy would be needed.
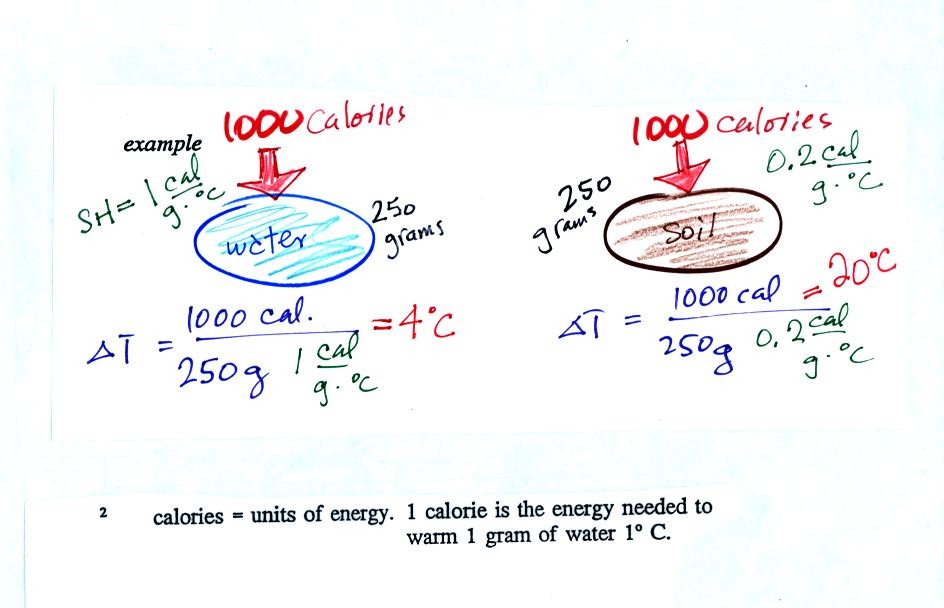
Here's an example that shows the effect of specific
heat. Equal
amounts of energy (note that calories are units of energy) are added to
equal masses of water and dirt. We use water and dirt in the
example because most of the earth's surface is either water or dirt.
Water has a higher specific heat than soil, it only warms up 4 C.
The soil warms up 20 C.
The following figure wasn't shown or
mentioned in class.
These different rates of warming of water and soil have important
climate implications.
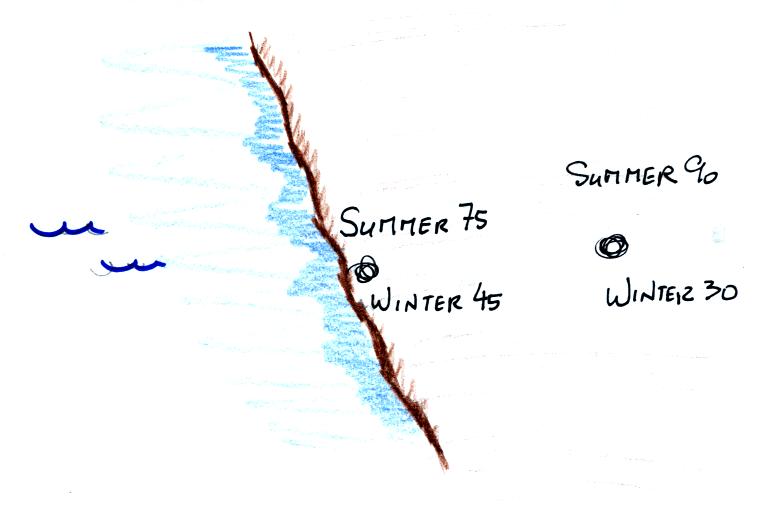
Oceans moderate the climate. Cities near a large body
of water won't warm as much in the summer and won't cool as much during
the winter compared to a city that is surrounded by land.
The city above on the
coast has a 30o F annual range of temperature. The
city further
inland (assumed to be at the same latitude and altitude) has an annual
range of 60o F. Note that both cities have the same 60o
F annual
mean temperature.
Proximity to land or water is one of three or
four factors that determine a region's climate. Latitude and
altitude also play important roles. This is discussed in Chapter
3 and will probably be a topic on a future Optional Homework Assignment.
We did a
short experiment in class. The object of the experiment was to
measure the latent heat of
vaporization of liquid nitrogen. That just means measuring the
amount of energy needed to evaporate a gram of liquid nitrogen.
The students that are doing Experiment #2 are measuring the latent heat
of fusion of ice, the energy needed to melt one gram of ice.
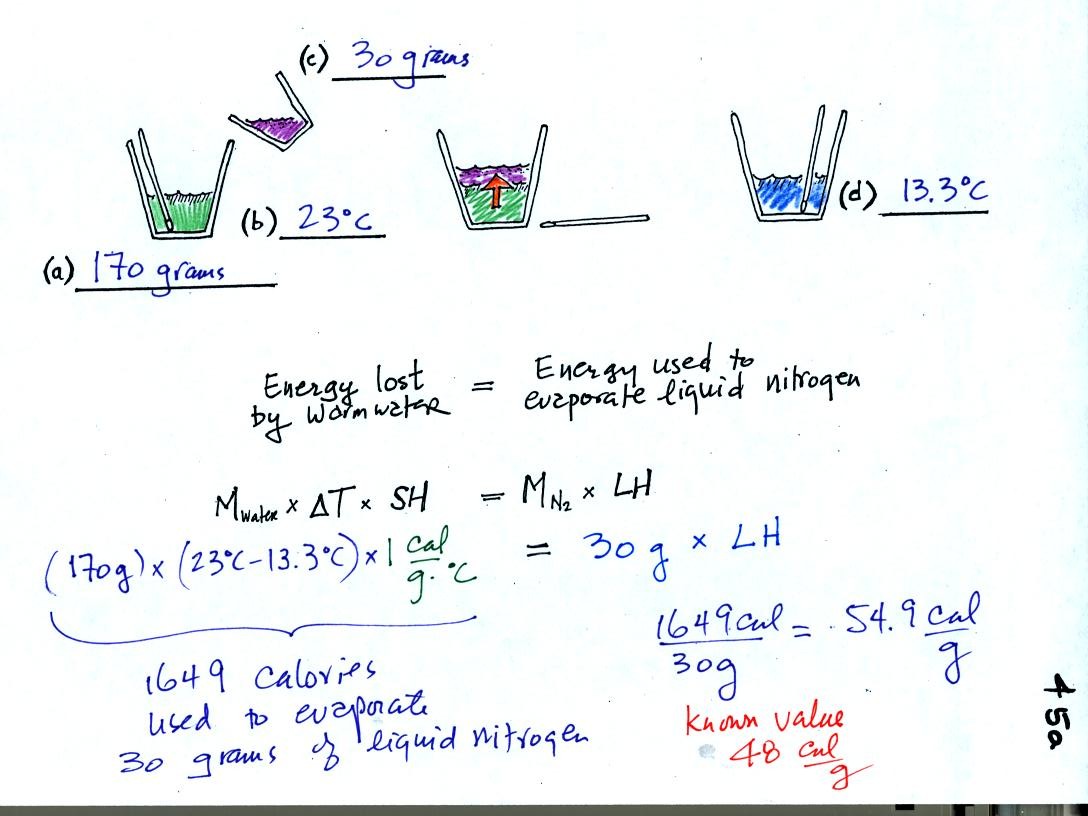
We poured some water into a styrofoam cup and weighed it
(170 grams) and measured its
temperature (23 C). Then we weighed out 30 grams of liquid
nitrogen into a second cup and poured it into the cup of water (after
having removed the thermometer). We waited until all the liquid
nitrogen had evaporated and remeasured the temperature of the water.
It takes energy to turn liquid nitrogen into nitrogen gas.
The needed energy came from the water (we assume that because the
experiment was performed in a styrofoam cup that the warm room air
didn't contribute any energy).
When energy was removed
from the water the water cooled to 13.3 C. Because we knew how
much water we started with, its temperature drop, and water's specific
heat we calculated how much
energy was taken from the water. That is the 1649 calorie
figure above. This was used to evaporate 30 grams of liquid
nitrogen. So we divided 1649 calories by 30 grams to get 54.9
calories needed per gram. That is our
measured value of the latent heat of vaporization of nitrogen.
The know value is 48 cal/g, so our measurement
was reasonably close.
The following material wasn't covered
in class on Friday.
When you add energy to something, the object warms. What exactly
is happening inside the material?

The figure above is on p. 46 in the photocopied Class
Notes. Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
The next figure might make the distinction between temperature (average
kinetic energy) and heat (total kinetic energy) clearer.
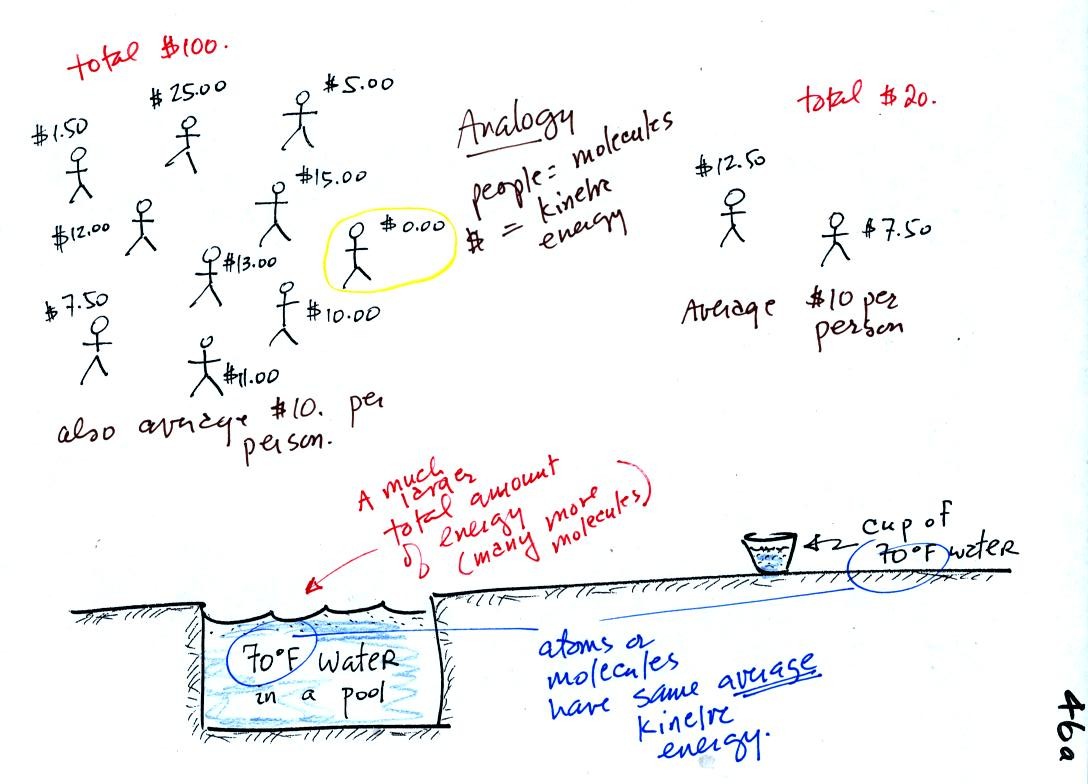
A cup of water and a pool of water both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So if you add together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people shown have the same average
amount
of money per person. The $100 held by the group at the left is
greater than the $20 total possessed by the smaller group of people on
the right.
You need to be careful what temperature scale you use when using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero. THe smallest kinetic energy you can have is zero
kinetic energy. There is no such thing as negative kinetic energy.

You should remember the temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and Kelvin scales. 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth.
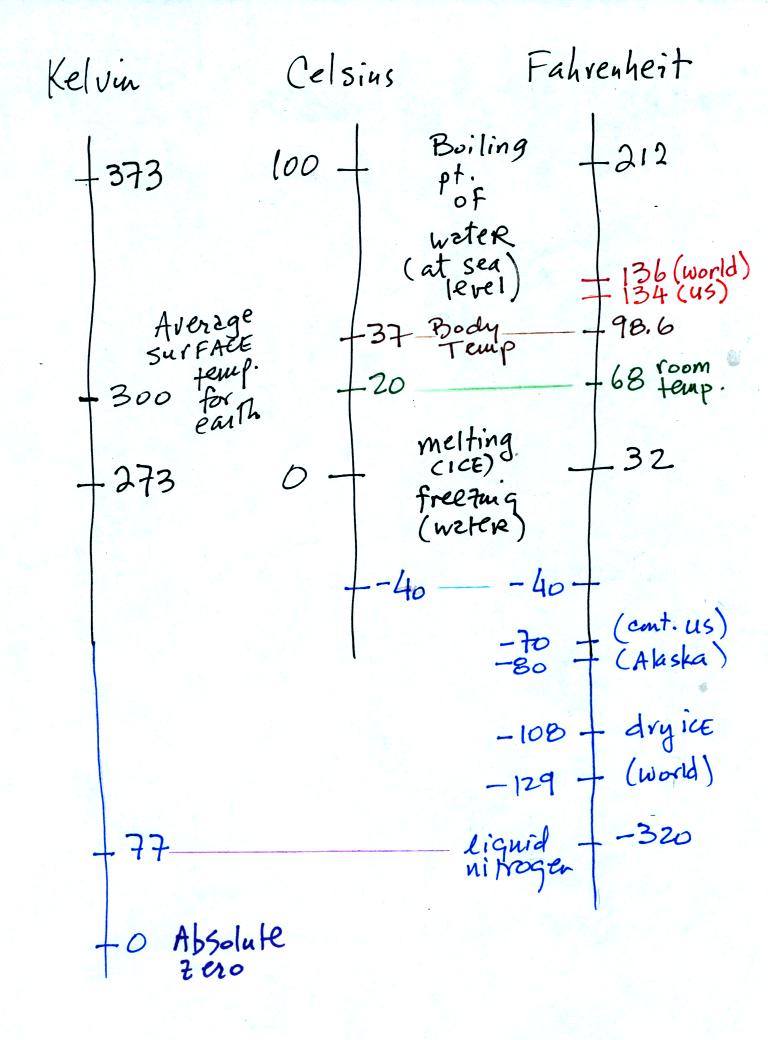
You certainly don't need to try to remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than being
near or
surrounded by ocean. You'll find more record high and low
temperature data on p. 58 and p. 61 in Chapter 3 of the text.
Precipitation records are shown on p. 358. Note that even liquid
nitrogen is still quite a bit warmer than absolute zero.














