Tuesday Oct. 30, 2007
The Experiment
#3 reports and the revised
Expt. #2 reports were collected today. It usually takes about one
week to grade the experiment reports.
Photocopies of the Quiz
#3 Study Guide
were distributed in class.
We'll
finish precipitation formation today (neither of the next two figures was
shown in class)
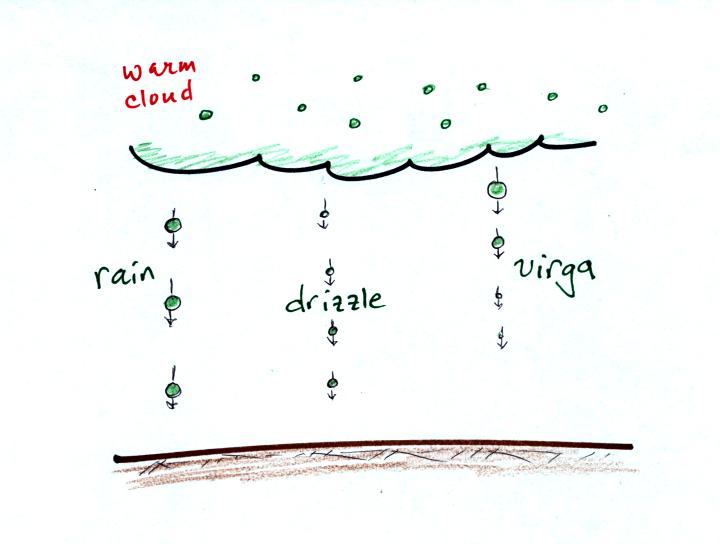
The collision coalescence process is pretty straight
forward. It
doesn't produce much of a variety in types of precipitation particles.
Looking ahead we are going to find that the ice crystal process is more
complex
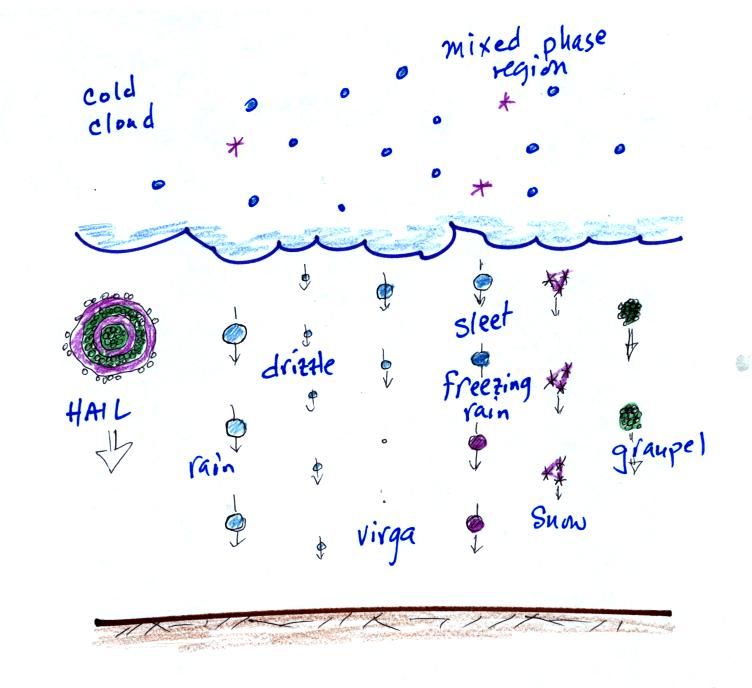
both in regards to what goes on inside the cloud and also in
the types
of precipitation that can fall from the cloud. Before we can
begin to understand how the ice crystal process works we need to learn
somethings about cold clouds.
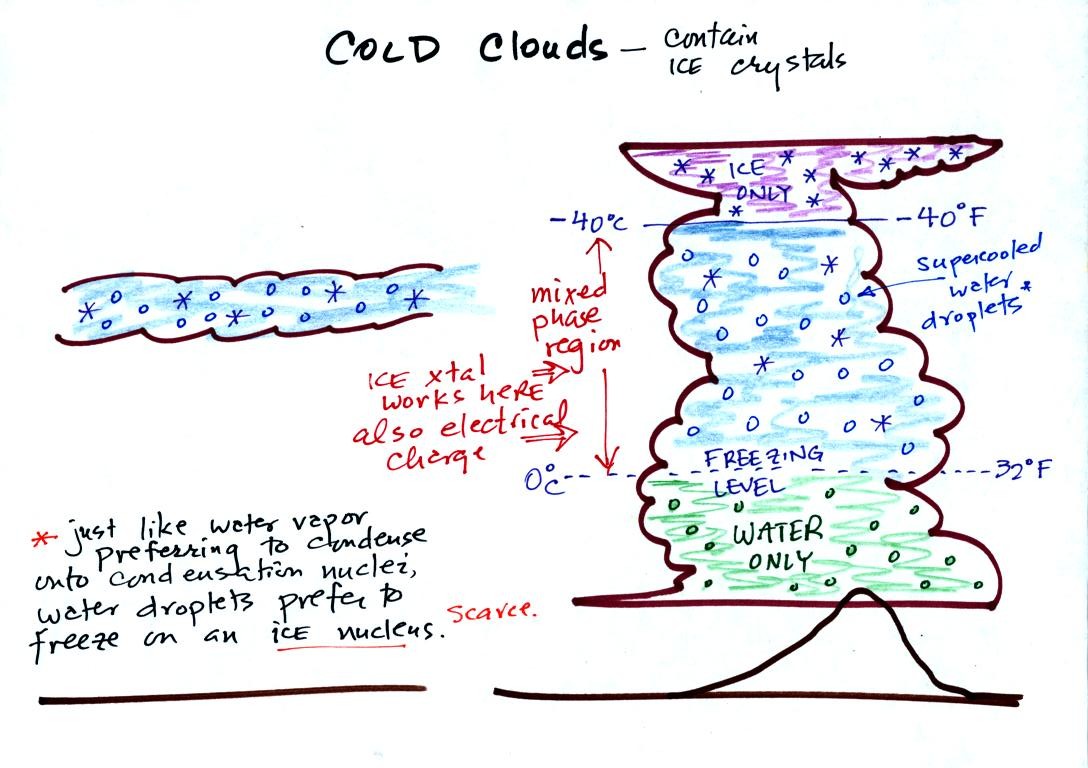
The bottom of the thunderstorm (shaded green) is warm enough
(warmer than freezing) to just
contain water
droplets. The top of the thunderstorm (colored purple) is colder
than
-40 C and just contains ice crystals. The interesting part of the
thunderstorm and the
nimbostratus cloud is the middle part (blue) that contains both
supercooled water
droplets (water that has
been cooled to below freezing but hasn't frozen) and ice
crystals.
This is called the mixed phase
region. This is where the ice crystal process will be able
to produce
precipitation. This is also where the electrical charge that
results in lightning is generated.
The supercooled water droplets aren't able to freeze even though
they
have been cooled below freezing. This is
because it is much
easier for small droplets of water to freeze onto an ice crystal
nucleus (just like it is easier for water vapor to condense onto
condensation nuclei rather than condensing and forming a small droplet
of pure water). Not just any material will work as an ice nucleus
however. The material must have
a crystalline structure that is like that of ice. You'll find
some additional discusssion of cold clouds on p. 94 in the photocopied
Classnotes.
Now we can begin to see how the ice crystal process works (the
discussion that follows is found on p. 101 in the Classnotes). There
are
a
couple of tricky parts.
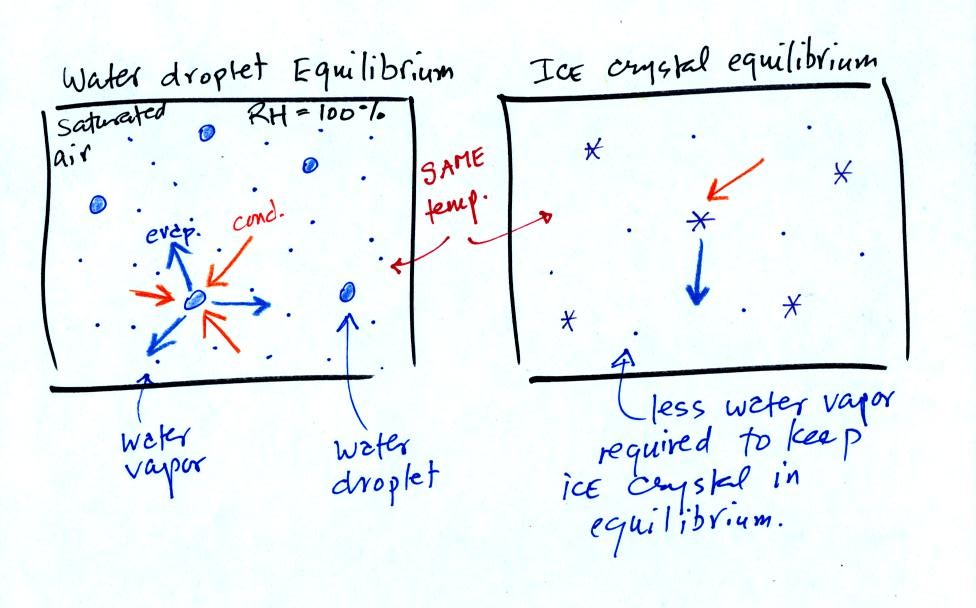
The left figure above
shows a water droplet in equilibrium with its surroundings..The droplet
is evaporating (the 3 blue arrows in the figure). The rate of
evaporation will depend on the temperature of the water droplet.
The droplet is surrounded by air that is saturated with water vapor
(the droplet is inside a cloud where the relative humidity is
100%). This means there is enough water vapor to be able to
supply 3 (orange) arrows of condensation.
The right figure shows what is required for an ice crystal (at the same
temperature) to be in equilibrium with its surroundings. First
the ice crystal won't evaporate as rapidly as the water droplet (only
one arrow is shown). Going from ice to water vapor is a bigger
jump than going from water to water vapor. There won't be as many
ice molecules with enough energy to make that jump. A sort of
analogous situation is shown in the figure below (many people could
jump up a 1 foot step, fewer people would be able to jump up a 3 foot
step).
To be in equilibrium only one arrow of condensation is needed.
There doesn't need to be as much water vapor in the air surrounding the
ice crystal to supply this lower rate of condensation.
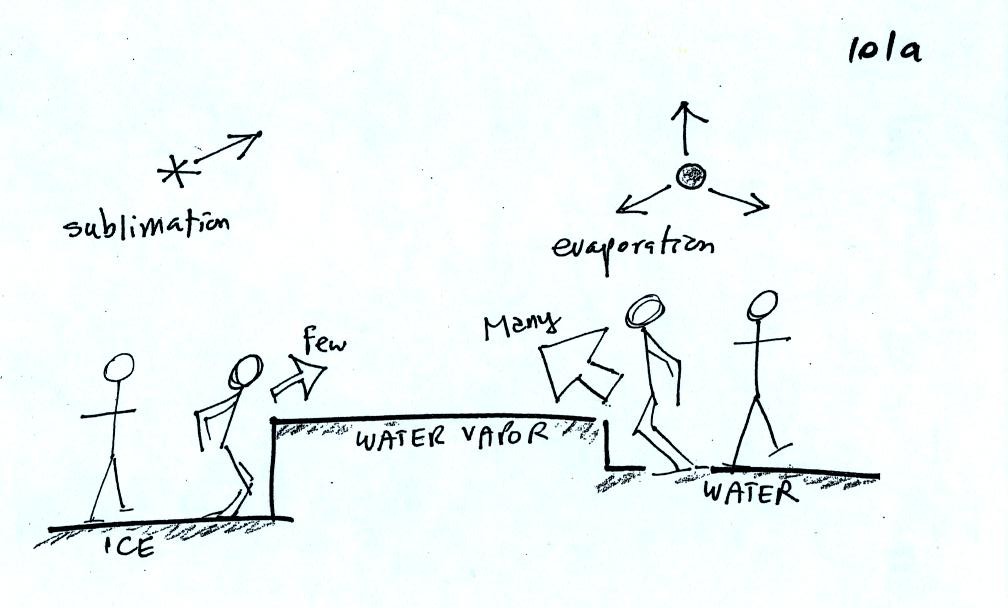
There are going to be fewer people able to make the big jump on the
left just as there are fewer ice molecules able to sublimated.
Going from water to water vapor is a "smaller jump" and more molecules
are able to do just as more people would be able to make the shorter
jump at right in the picture above.
Now what happens in the mixed phase region of a cold cloud
is that
ice crystals find themselves in the very moist surroundings needed for
water droplet equilibrium. This is shown below.
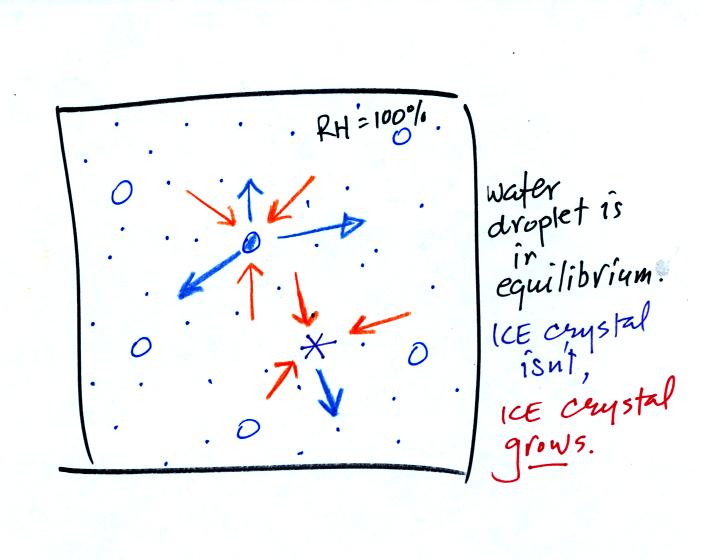
The water droplet is in equilibrium (3 arrows of evaporation
and 3
arrows of condensation) with the surroundings. The ice crystal is
evaporating more slowly than the water droplet. Because the ice
crystal is in the same surroundings as the water droplet water vapor
will be condensing onto the ice crystal at the same rate as onto the
water droplet. The ice crystal isn't in equilibrium, condensation
exceeds evaporation and the ice crystal will grow.
The equal rates of
condensation are shown in the figure below using the
earlier analogy.
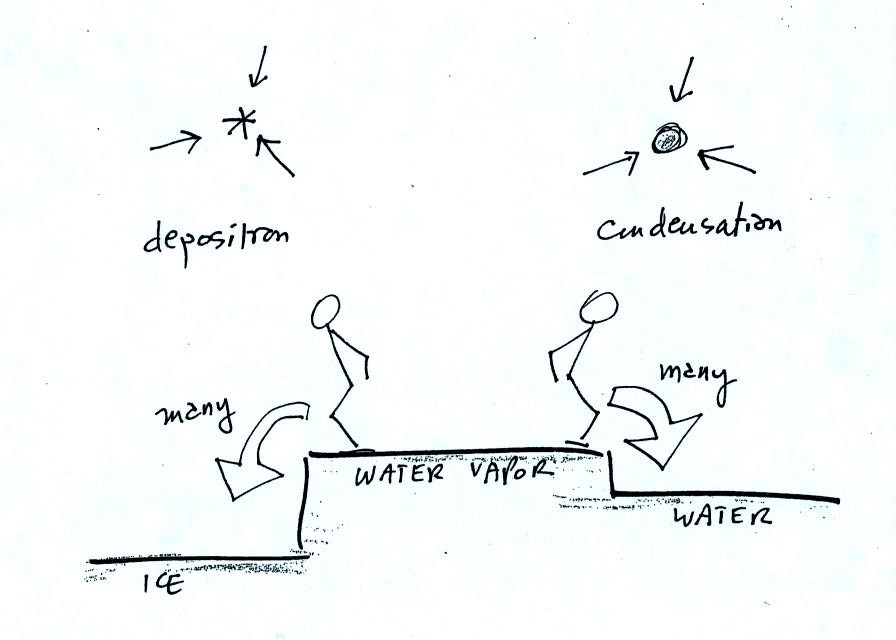
The
growing ice crystal is just the start. Here's what can happen
next.
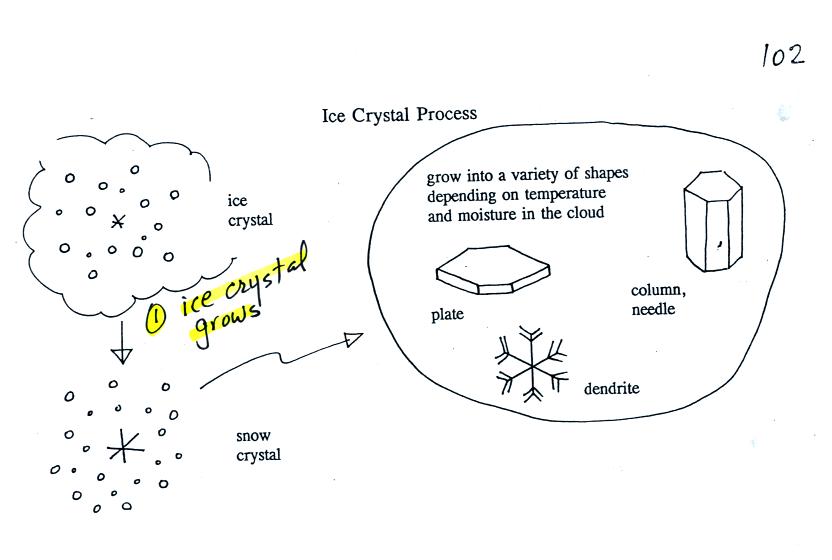
Once an ice crystal has grown a little bit it becomes a snow
crystal. Snow crystals can have a variety of shapes (called
crystal habits) depending on the conditions
(temperature and moisture) in the cloud. Dendrites are the most
common because they form where there is the most moisture available for
growth. With more raw material available it makes sense there
would be more of this particular snow crystal shape.
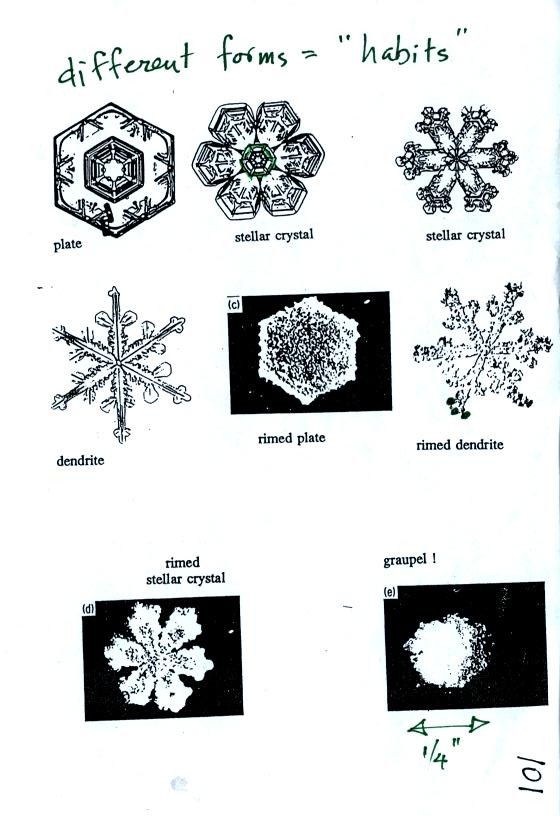
Here are some actual photographs of snow crystals (taken
with a
microscope). Snow crystals are usually 100 or a few 100s of
micrometers in diameter (tenths of a millimeter in diameter).
You'll find some much better photographs and a pile of addtional
information about snow crystals at www.snowcrystals.com
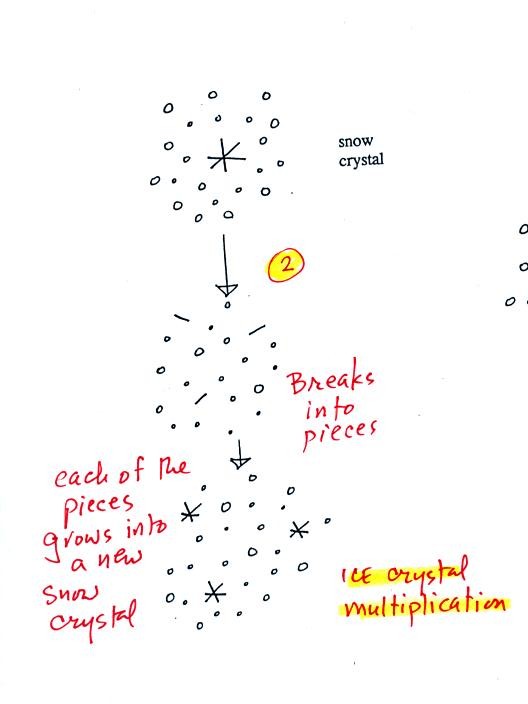
A variety of things can happen once a snow crystal
forms. First
it can break into pieces, then each of the pieces can grow into a new
snow crystal. Because snow crystals are otherwise in rather short
supply, ice
crystal multiplication is a way of increasing the amount of
precipitation that ultimately
falls from the cloud.
This is incidentally the idea behind cloud seeding, to increase the
number of ice crystals and hopefully the amount of precipitation.
Silver iodide is often
used and is
one of the relatively rare materials that can act as an ice crystal
nucleus. However it is possible to "overseed" a cloud and end up
with too many ice crystals. Then they all fight for a limited
amount of water vapor and, as a result, do not get very big.
Overseeding a cloud could decrease the precipitation from a cloud.
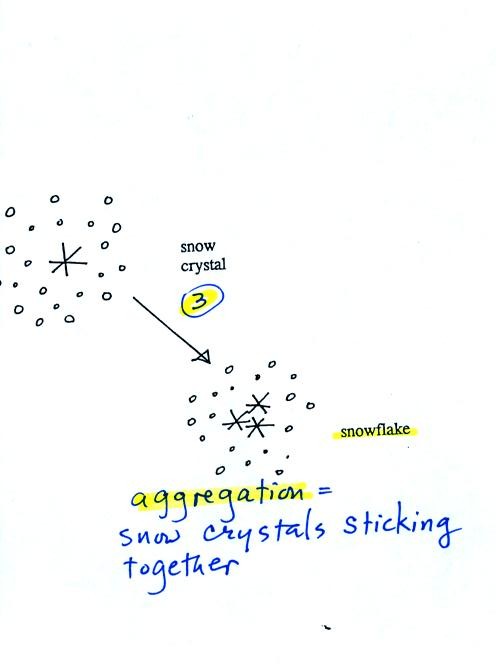
Several snow crystals can collide and stick together to form
a
snowflake. Snow crystals are small, a few tenths of a millimeter
across. Snowflakes can be much larger and are made up of many
snow crystals stuck together. The sticking together or clumping
together of snow
crystals is called aggregation.
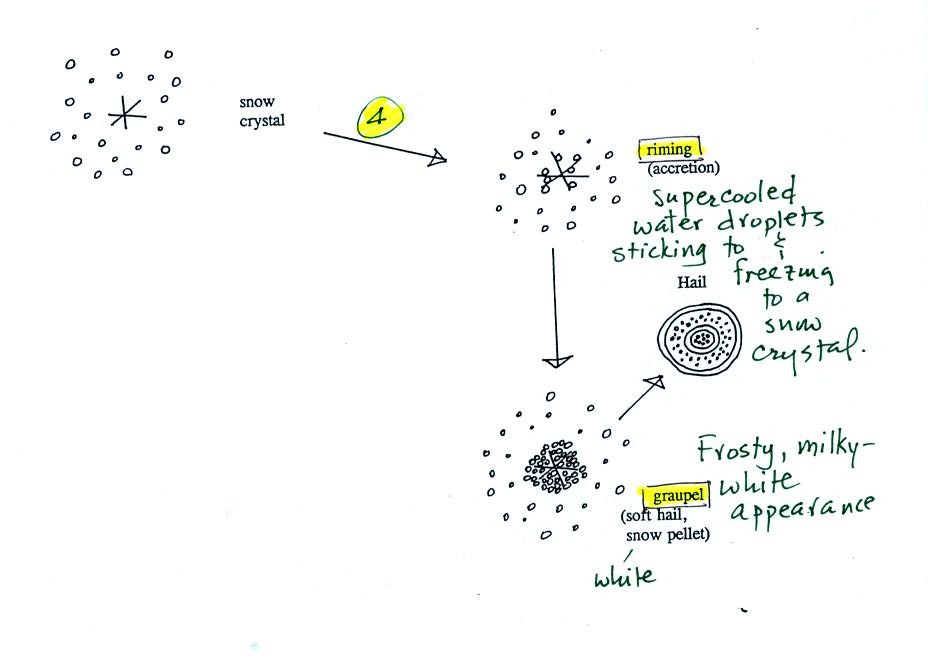
Snow crystals can collide with supercooled water
droplets. The
water droplets may stick and freeze to the snow crystal. This
process is called riming or accretion (note it is really the same idea
as collision and coalescence). If a snow crystal collides with
enough water droplets it can be completely covered with ice. The
resulting particle is called graupel (or snow pellets). Graupel
is sometimes mistaken for hail and is called soft hail or snow
pellets. Rime ice
has a frosty milky white appearance. A graupel particle resembles
a miniature snow ball. Graupel particles often serve as the
nucleus for a hailstone.

Hail forms in thunderstorms with very strong updrafts.
In the
figure above the hailstone starts with a graupel particle (colored
green to represent rime ice). The graupel falls or gets carried
into a part of the cloud where it collides with a large number of
supercooled water droplets which stick to the graupel but don't
immediately freeze. The graupel gets coated with a layer of
water (blue). The particle then moves into a colder part of the
cloud
and the water layer freeze producing a layer of clear ice (the clear
ice, colored violet, has a distinctly different appearance from the
milky white rime
ice). In Tucson this is often the only example of hail that you
will see: a graupel
particle core with a single layer of clear ice.
In the severe thunderstorms in the Central Plains, the hailstone can
pick up a new layer of rime ice,
followed by another layer of water which subsequently freezes to
produce a layer of clear ice.
This cycle can repeat several times; large hailstones can be composed
of many alternating layers of rime and
clear ice. An unusually large hailstone (around 3 inches in
diameter) has been cut in
half to show (below) the different layers of ice.
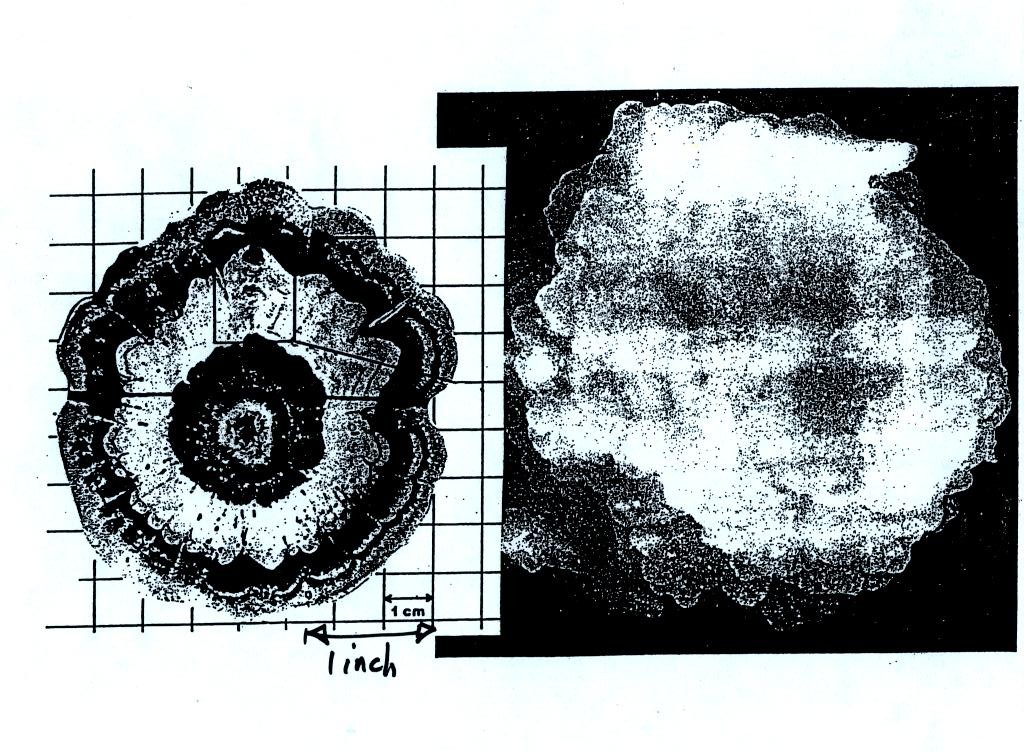
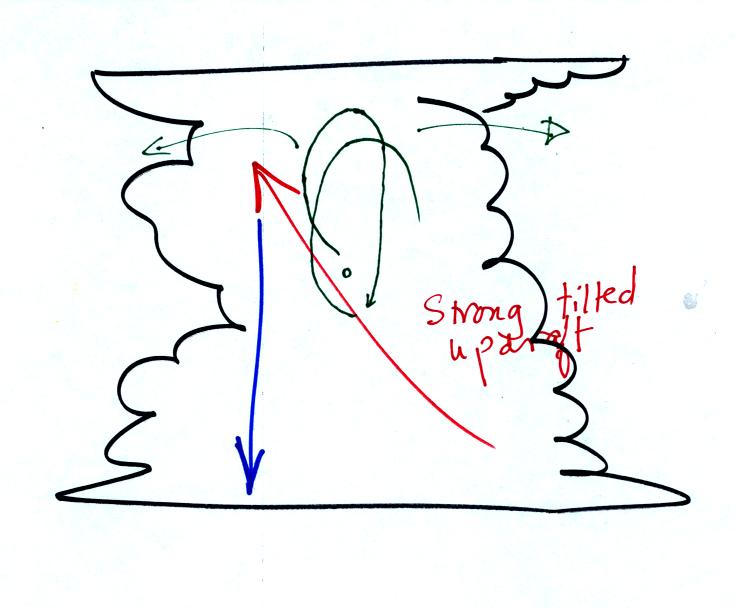
The largest hailstones are produced in strong thunderstorms with tilted
updrafts (the updraft may also spin). Complex air motions inside
the cloud support the hailstone and move it through different cloud
environments so that it
can grow and acquire the layers of rime and clear ice.
The ice
crystal process can produce a variety of precipitation particles inside
the cloud. Further changes can occur once the particle falls from
the cloud.

In the example above at left the particle first melts and
then
evaporates before reaching the ground. Rain that evaporates
before reaching the ground is called virga.
A similar thing can
happen with snow crystals or snow flakes. They sublimate away;
the streamers of falling precipitation are called fall streaks (as far
as I'm concerned you can use the name virga for this process since
it is the
same overall idea). You'll see white streamers falling from
cirrus clouds fairly often.

The frozen precipitation particles produced by the ice
crystal process
(graupel or snow) can melt before reaching the ground. This would
be rain (or drizzle if the drops are small). Rain in most
locations at most times of the year starts out as frozen precipitation
(even in Tucson in the summer).
If you are on a mountain top you might see some of the frozen
precipitation before it melts. You might see graupel falling from
a summer thunderstorm, for example, while the people in the valley only
observe rain.

Sometimes the frozen precipitation will melt and then fall
into a thick
layer of cold air and refreeze. The resulting particle is called
sleet (or ice pellets). The clear ice in sleet is noticeably
different from the frosty, milky white, rime ice in graupel.
Rain that falls into a shallow cold air layer and freezes after
reaching the ground is called freezing rain. It is nearly
impossible to drive during one of these "ice storms." Sometimes
the coating of ice is heavy enough that branches on trees are broken
and power lines are brought down. It sometimes takes several days
for power to be restored.
Satellite
photographs are a good way of observing clouds
(especially out over the ocean). Using both
visible and
infrared light satellite photographs, you can get a good idea of cloud
type. However satellite
photographs don't really tell you whether a cloud is producing
precipitation or not. For that you need radar.
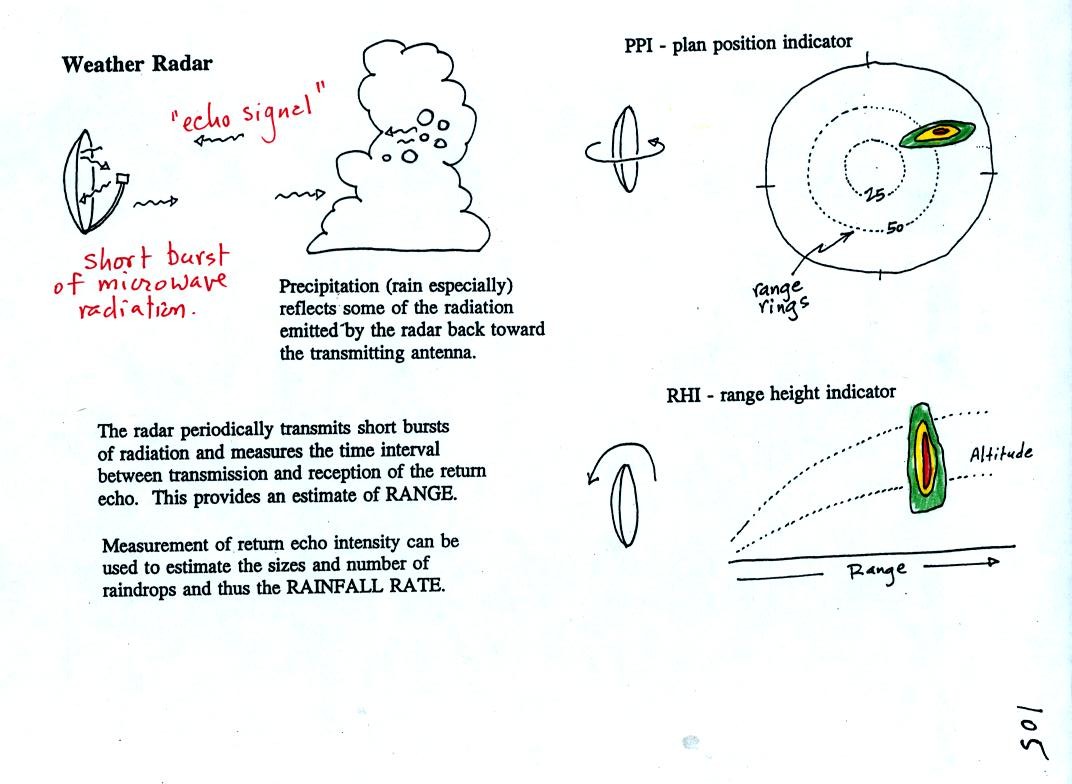
An ordinary radar periodically transmits a short burst of
microwave
radiation. This radiation penetrates a cloud but is reflected by
precipitation particles. The radar keeps track of what direction
the antenna is pointing and determines how long it takes for a signal
to go out and return. The radar also measures the strength of the
return signal. Conventional radar can thus locate the
precipitation and provide an estimate of its intensity.
The radar antenna slowly spins as it is transmitting so it
scans a full
360 degrees in a minute or two.
Information from a single radar or a combination of data from many
radars are drawn on weather maps (the PPI display above shows the data
from a single radar, the radar would be at the center of the
picture). This would show where precipitation is occurring.
The radar data is often combined with satellite photographs.
Colors are used to indicate the intensity of the precipitation.
Yellows, oranges and reds generally indicate the heaviest precipitation
(often coming from thunderstorms).
In research the radar can be used to scan vertically through a storm,
this produces an RHI display.
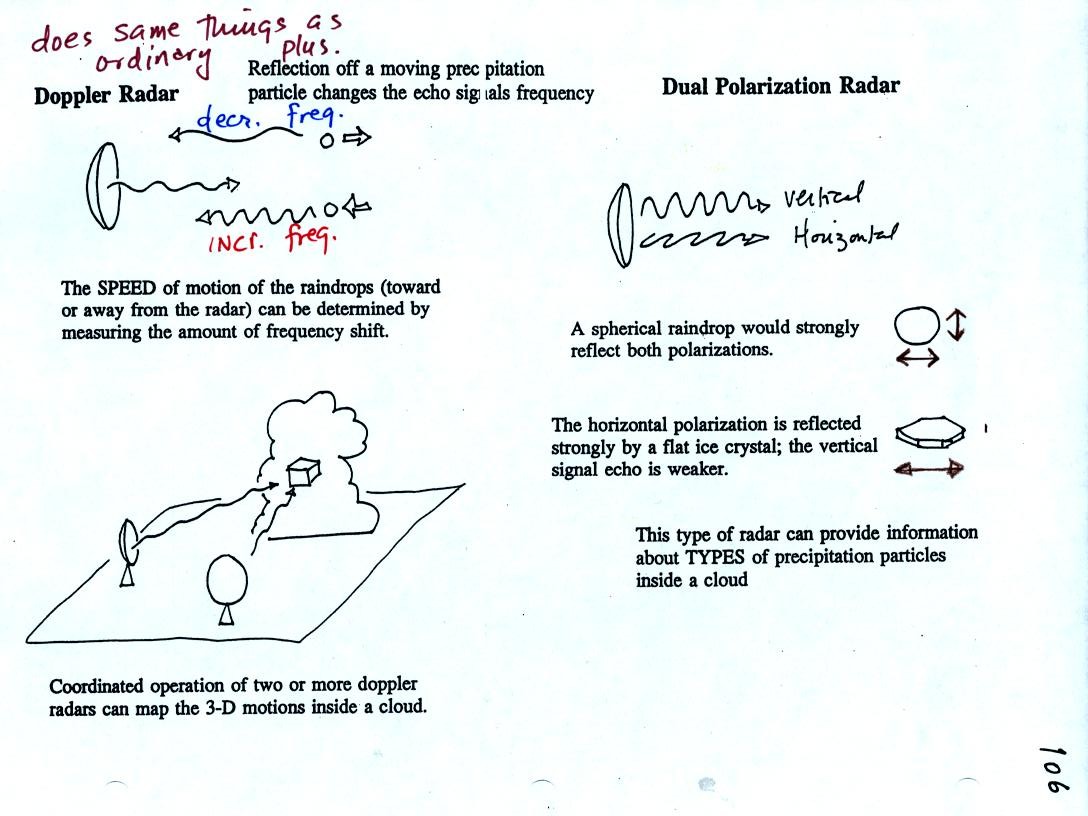
By detecting changes in the frequency of the reflected
signal, a
doppler radar can measure the speed at which precipitation particles
are moving toward or away from a radar antenna. By combining data
from 2 or more radars (and some complicated computer processing),
three-dimensional wind motions inside a cloud can be mapped out.
Doppler radars can detect a rotating thunderstorm updraft (a
mesocyclone) that could indicate a thunderstorm capable of producing
tornadoes. Small mobile doppler radars are being used to try to
measure wind speeds in tornadoes. Police use doppler radar to
measure the speeds of automobiles on the highway.
Dual polarization radar is a research tool that can be used
to learn something about the kinds of
precipitation particles inside a cloud.



















