Thu., Sept. 6, 2007
The first 1S1P assignment of the semester
was mentioned briefly. You can choose from the topics listed and
write up to 2 reports. Reports are due on Tue., Sept. 18.
The first optional assignment of the
semester was handed out in class. Assignments are due at the
beginning of class on Thu., Sept. 13.
We had a quick look at satellite imagery and the predicted path of
Tropical Depression Henriette. Henriette was bringing cloudy
skies, cooler temperatures, and scattered showers to southern
Arizona. The center of the remnants of Henriette was expected to
pass to the east of Tucson, that is where the heavier rains were
expected.
Note a new reading assignment was
posted Friday after class on Thursday.
In this
class we will sometimes "beat topics to death." We will do
that today in trying to understand how mercury barometers work.
You will find most of what follows on p. 29 in the photocopied Class
Notes. The pictures below
are hopefully clearer and more carefully drawn versions of what were
drawn in class.

The instrument above ( a u-shaped glass tube filled with a
liquid of some kind) is a manometer and can be used to measure pressure
difference. The
two ends of the tube are open so that air can get inside and air
pressure can press on the liquid. Given that the liquid levels on
the two sides of the manometer
are equal, what could you about PL and PR?
The liquid can slosh back and
forth just like the pans on a balance can move up and down. A
manometer really behaves just like a pan balance.
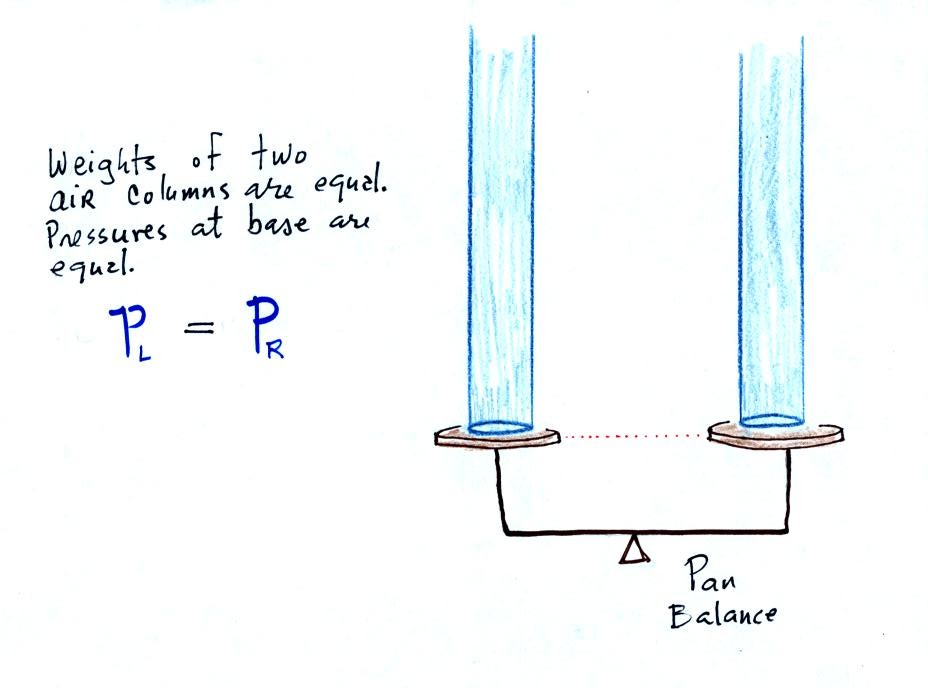
PL and PR are equal (note
you don't really know what either pressure is just that they are equal).
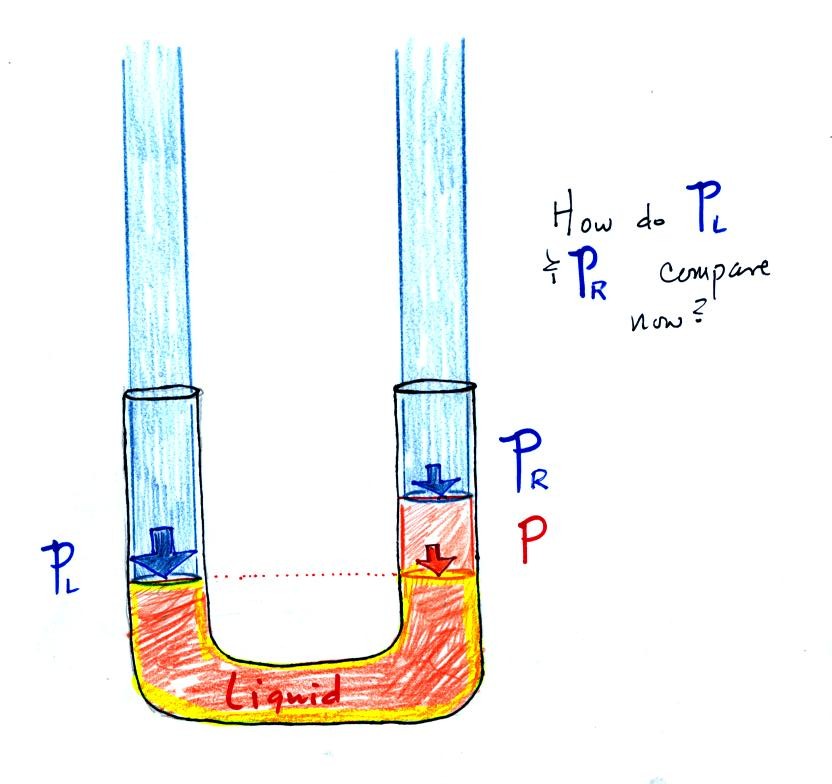
Now the situation is a little different, the
liquid levels
are no
longer equal. You probably realize that the air pressure on the
left, PL, is a little higher than the air pressure on the
right,
PR. PL is now being balanced by PR
+ P acting together. P
is the pressure produced by the extra fluid on the right hand side of
the manometer (the fluid that lies above the dotted line). The
height of the column of extra
liquid provides a measure of the difference between PL and PR.
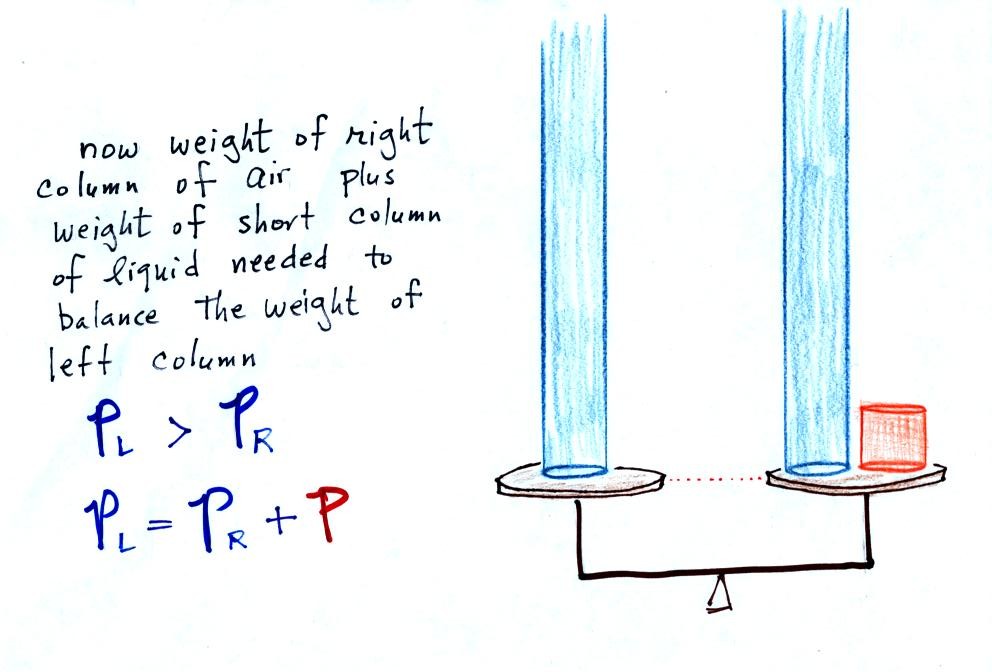
Next we will go an extreme and close off the right hand side of the
manometer.
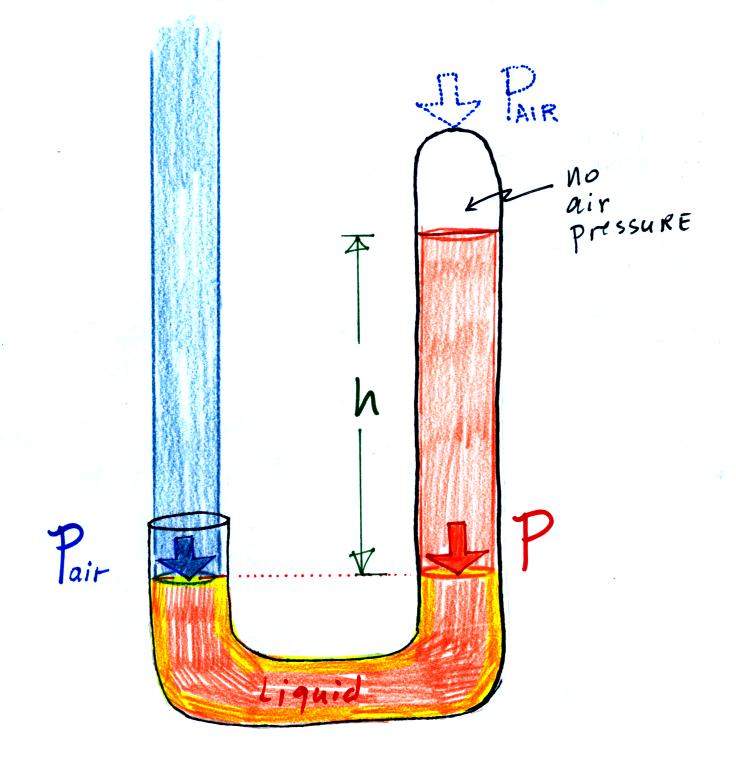
Air pressure can't get into the right tube any
more. Now at the level of the dotted line the balance is between
Pair and P (pressure by the extra liquid on the
right). If
Pair changes, the height of the right column, h, will
change. You now have a barometer, an instrument that can measure
and monitor theatmospheric pressure.
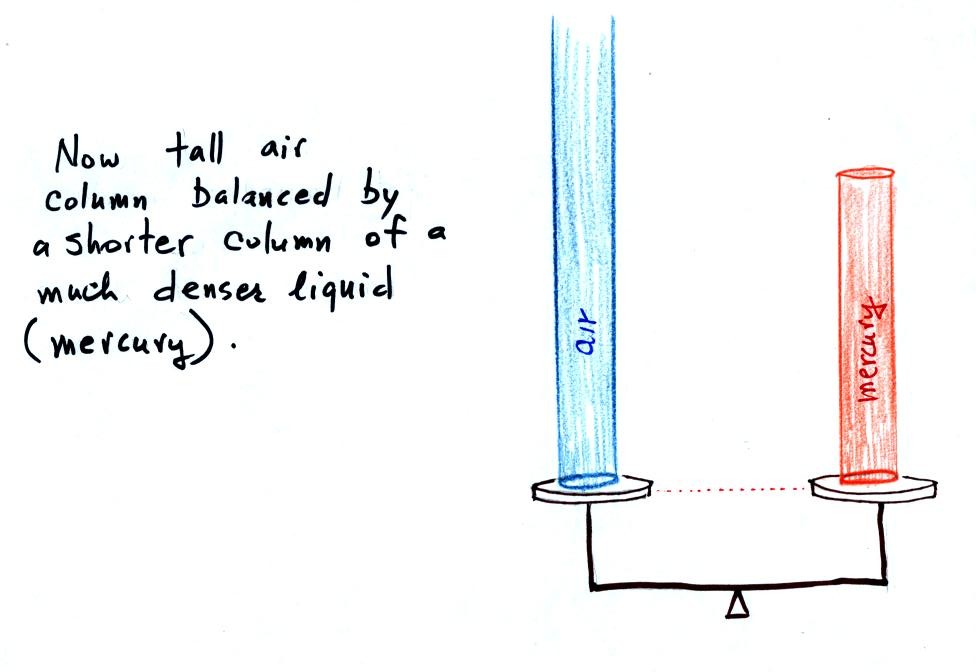
Barometers like this are usually filled with mercury. Mercury is
a liquid. You need a liquid that can slosh back and forth in
response to changes in air pressure. Mercury is also dense which
means the barometer won't need to be as tall as if you used something
like water. A water barometer would need to be over 30 feet
tall. With mercury you will need only a 30 inch tall column to
balance the weight of the atmosphere at sea level under normal
conditions (remember the 30 inches of mercury pressure units mentioned
earlier). Mercury also has a low rate of
evaporation so you don't have much mercury gas at the top of the right
tube.

Finally here is a more conventional barometer design.
The bowl of
mercury is usually covered in such a way that it can sense changes in
pressure but not evaporate and fill the room with poisonous mercury
vapor.

The figure above (p. 30 in the photocopied Class Notes) first
shows average sea level pressure values (1000 mb or 30 inches of
mercury are close enough in this class). Sea level pressures
usual fall between 950 mb and 1050 mb. Record high sea level
pressure values occur during cold weather. Record low pressure
values have all been set by intense hurricanes (the record setting low
pressure is the reason these storms were so intense)
.
Next we
looked at some of the historical events listed on pps 31 and 32 in the
photocopied class notes. The barometer was invented in
the mid-1600s following the discovery by Galileo that air had
weight. Note (at the botom of p. 31) the amount clothing that
Hawthorne Charles Grey wore on this trip to 28,510 feet altitude (in an
open balloon cabin).

Grey would die on a later trip to 42,470 feet when he ran out of
oxygen. We watched a short video recalling Auguste
Piccard's first flight into the stratosphere by balloon (May 27,
1931). The video segment was from a PBS program titled "The
Adventurers."

The
remainder of the period was devoted to the Practice Quiz. Graded
quizzes will be returned next Tuesday.









