Thursday Sept. 27, 2007
A new Optional Assignment was handed out. It will be due at
the
beginning of class next Monday, Oct. 1.
A couple of new Reading Assignments have also been posted.
We spent
quite a bit of time at the start of class looking at some material
(energy transport by conduction and convection and some practical
applications) that was not discussed in class on Tuesday but was
nonetheless added to the end of the Tuesday notes.
You might have a look at that material if you haven't already done so.
Then we had a look at energy
transport in the form of latent heat. This is the second most
important
energy transport process (second only to electromagnetic
radiation). This process is sometimes a little hard to visualize
or understand because the energy is "hidden" in water vapor or water.
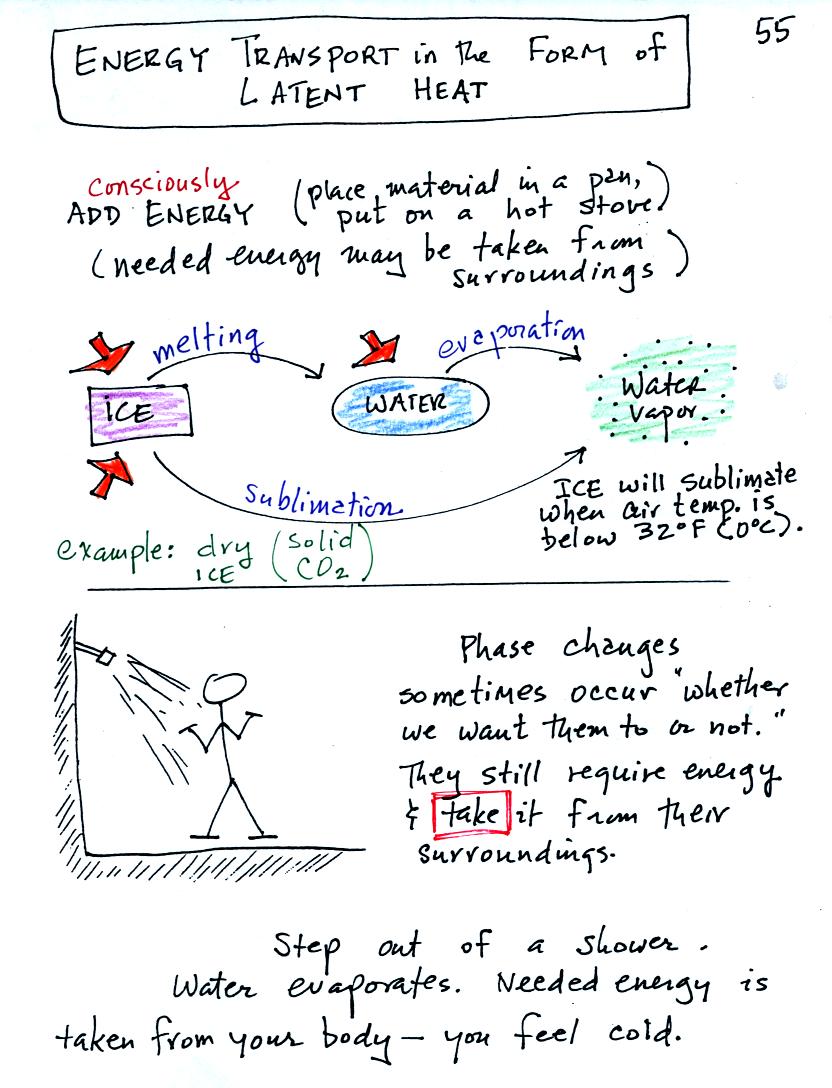
Latent heat energy transport is associated with changes of
phase (solid to liquid, water to water vapor, that sort of thing) A
solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change (dry ice
sublimates when placed in a warm room, it turns directly from solid
carbon dioxide to gaseous carbon dioxide).
In
each case energy must be added to the material changing phase.
You can consciously add or supply the energy (such as when you put
water in a
pan and put the pan on a hot stove) or the needed energy will be
taken from the surroundings (from your body when you step out of a
shower in the morning).
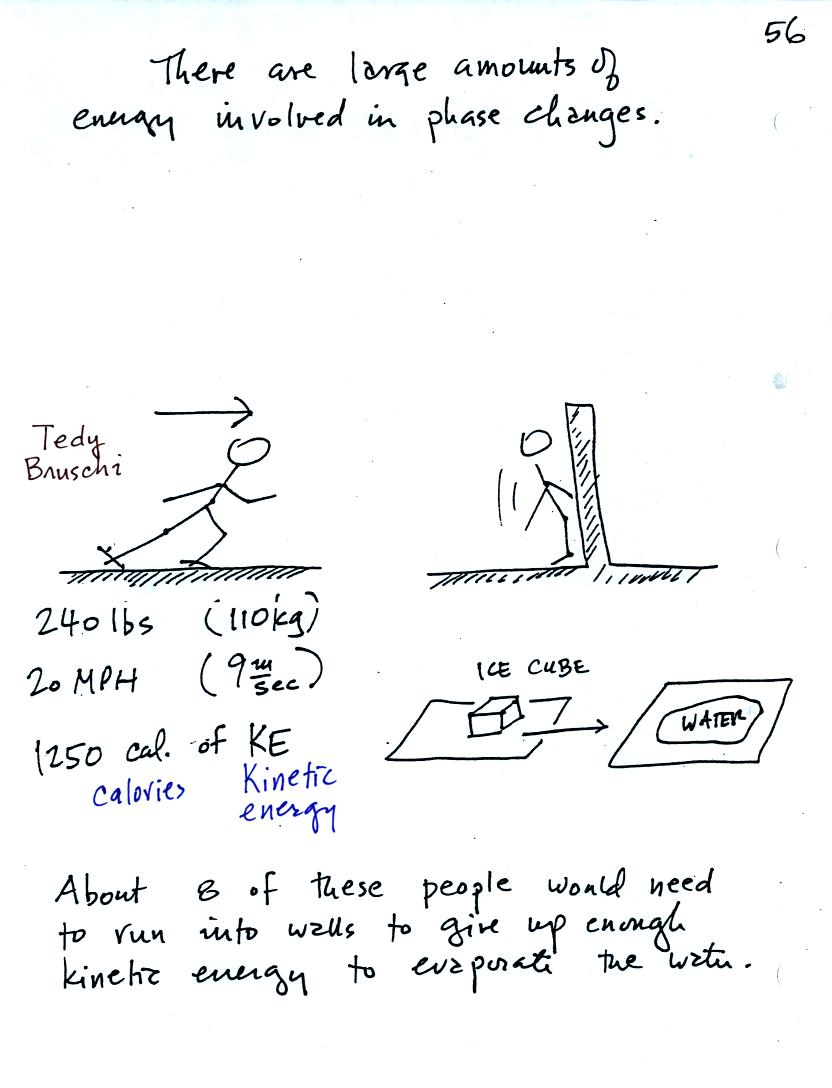
A 240 pound man (or woman) running at 20 MPH has just enough
kinetic energy (if you could somehow capture it) to
be able to melt an ordinary ice cube. It would take 8 people to
evaporate the resulting water.

You can consciously remove energy from water vapor to make
it
condense
or from water to cause it to free (you could put water in a
freezer; energy would flow from the relatively warm water to the
colder surroundings). Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm). Note the orange energy arrows have
turned around and are pointing from the material toward the
surroundings.
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more
rapidly. The condensation may actually be the dominant process.
The small figure at the bottom of the picture above shows that when a
teaspoon or two of water freezes and makes a single ice cube energy is
given off. It's not just a little bit of energy, it is the
kinetic energy that a 240 pound football player running 20 MPH would
have.
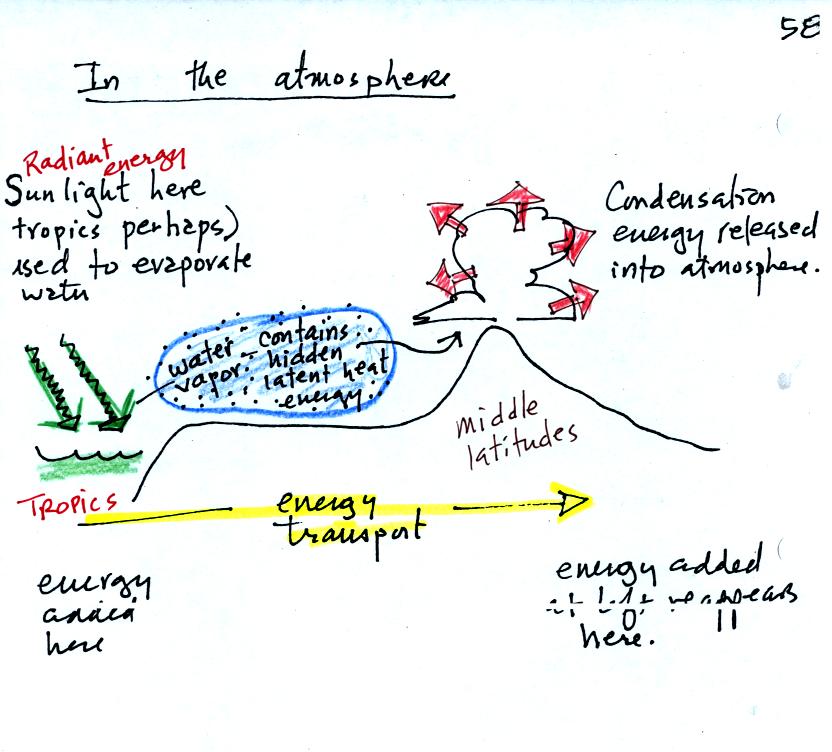
The story starts at left in the
tropics where there is often an abundance or surplus of energy;
sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy with it. This hidden energy
reappears when something (air running into a mountain and rising,
expanding, and cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in Tucson.
A piece of
dry ice was passed around in class. Dry ice is solid carbon
dioxide and is very cold. Dry ice sublimates, it changes directly
from solid carbon dioxide to carbon dioxide gas. Energy must be
added to the dry ice in order for it to sublimate. The figure
below (not shown in class)
shows that all three energy transport processes play a part.
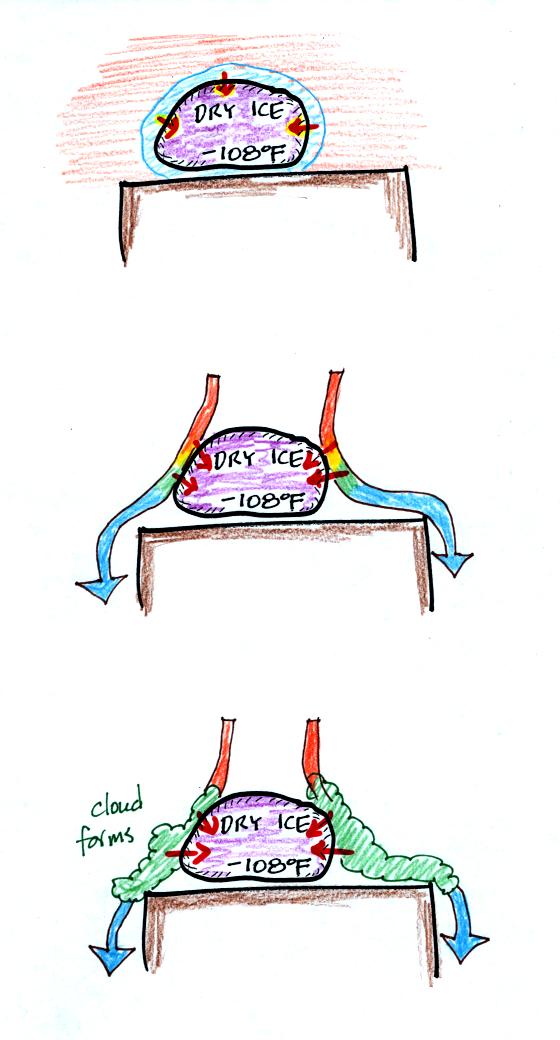
In the top figure, conduction transports energy from a thin
layer of
warm air in contact with the dry ice to the dry ice.
In the middle figure warm air coming into contact with the dry ice
looses energy and cools. This cool air sinks and is replaced by
warmer air. This process, free convection, also transports energy
to the dry ice.
Finally in the bottom figure, air that comes into contact with the dry
ice is cooled to the dew point and a cloud forms. Condensation
releases energy that flows into the dry ice.
A
bottle
containing solid iodine crystals was also passed around class together
with a bottle filled only with air. Iodine sublimates. What
is unusual however is that the iodine gas is visible. The bottle
containing the iodine has a just barely visible pink or purple
color. You can see a
picture of iodine and iodine gas at the webelements.com website.
We'll
spend the next couple of class periods on electromagnetic
radiation. It is the most important energy transport process
because it can travel through empty space.
To really understand EM radiation you need to understand electric
fields. To understand electric fields we need to quickly review
static electricity.
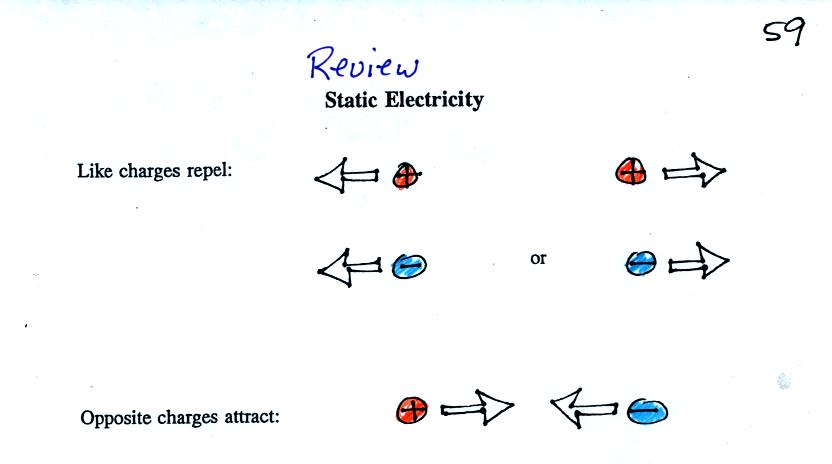
An electrical force is produced when two charged objects are
placed
next to each other. The force can be repulsive or attractive.
We used a
sweater (acrylic fiber and wool) and two balloons to demonstrate the
rules above.
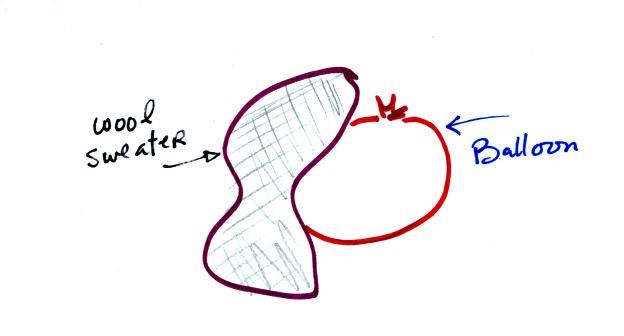
We rubbed two balloons with a sweater containing wool.
The
balloons (and the sweater) became electrically charged (the balloons
had one polarity of charge, the sweater had the other).
We didn't know what charge
the balloons carried just that they both had the same charge.
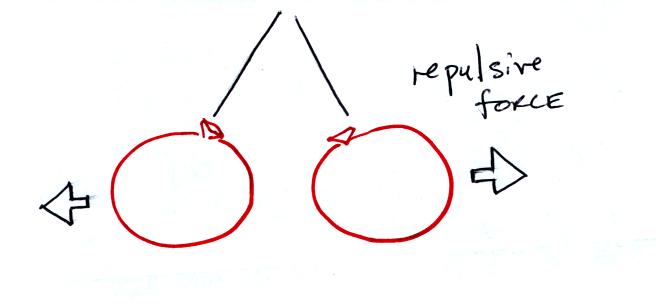
If you bring the balloons close to each other they are
pushed apart by
a repulsive electrical force.
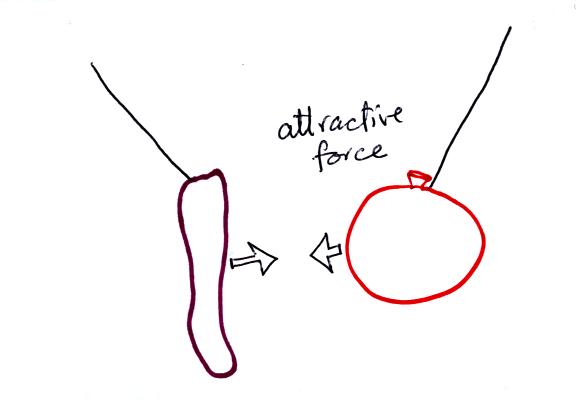
The sweater and the balloon carry opposite charges. IF
they are
brought together they experience an attractive electrical force.
The next
figure is from the bottom of p. 59 in the photocopied class notes.
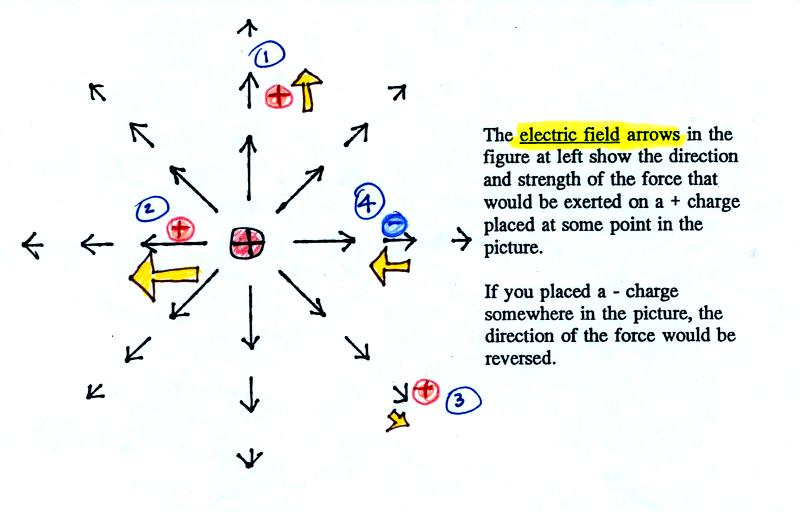
The balloons can help you understand
the picture above. Imagine placing one of the balloons at the
center of the picture and assume that it is positively charged.
The second balloon is placed at various positions (1, 2, and 3) around
the central balloon. The arrows in the picture are the electric
field. They give the direction and strength of the force that
would be exerted on the second positive charge. At Position 1,
for example a positively charged balloon would be pushed upward (by the
+
charge on the center balloon) with moderate
force. At Position 2 the force would point toward the left
but the force is stronger than at Point 1 because Position 2 is closer
to the center
charge. At Position 3 the charge is pushed toward the lower
right with a
weak force.
You can also use the electric field arrows to figure out what would
happen to a negative charge. The direction of the force is
reversed. A negative charge at Point 4 would be pulled in toward
the center
positive charge with moderate force.
The figures on p. 60 in the photocopied class
notes have been redrawn
below for clarity.

We imagine turning on a source of EM radiation and then a
short time
later we take a snapshot. The EM radiation is a wavy pattern of
electric and magnetic field arrows. We'll ignore the magnetic
field lines. The E field lines sometimes point up, sometimes
down. The pattern of electric field arrows repeats itself.
Note the + charge near the right side of the picture. At the time
this
picture was taken the EM radiation exerts a fairly strong upward force
on the + charge.
Textbooks often represent EM radiation with a wavy line like shown
above. But what does that represent?
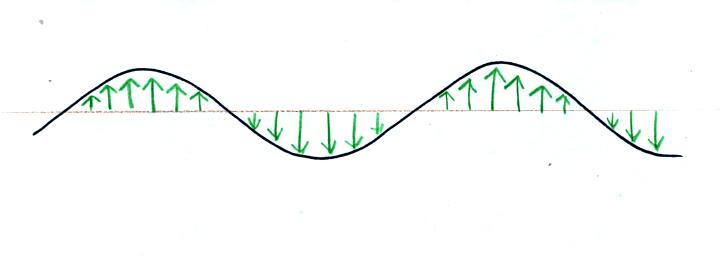
The wavy line just connects the tips of a bunch of electric
field
arrows.
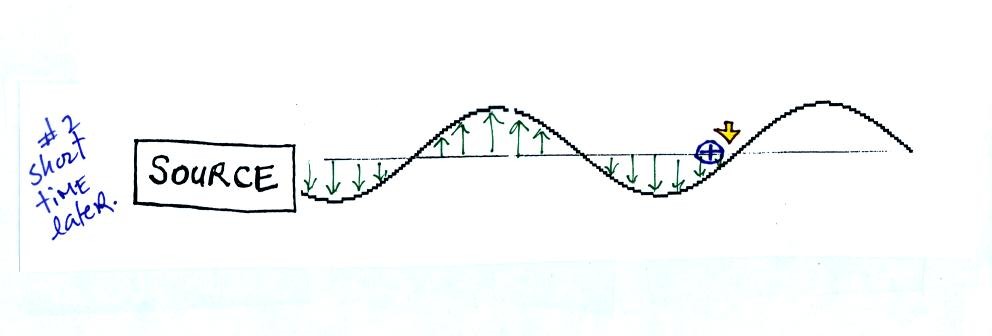
This picture was taken a short time after the first snapshot
when
the radiation
had
traveled a little further to the right. The EM radiation now
exerts a somewhat weaker downward force on the + charge.
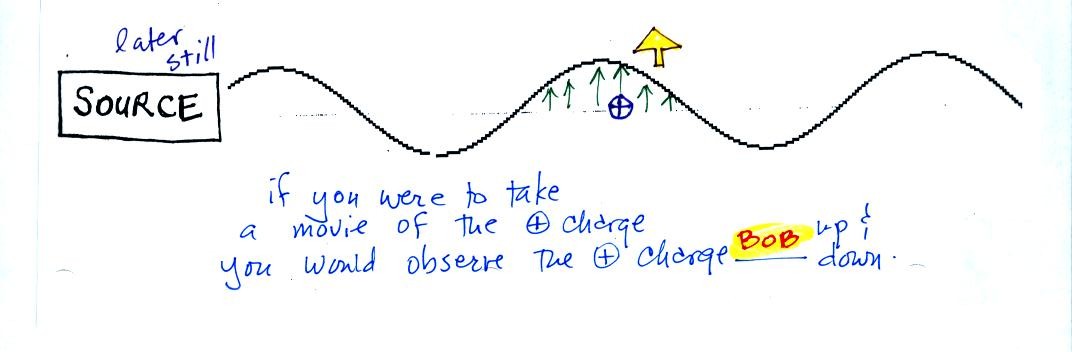
The + charge is now being pushed upward again. A movie
of
the +
charge, rather than just a series of snapshots, would show the charge
bobbing up and down much like a swimmer in the
ocean would do as waves passed by. (the
picture of the swimmer wasn't shown in class)

The wavy pattern used to
depict EM radiation can be described spatially in terms of its
wavelength,
the distance between identical points on the pattern. By
spatially we mean you look at different parts of the radiation at one
particular time.
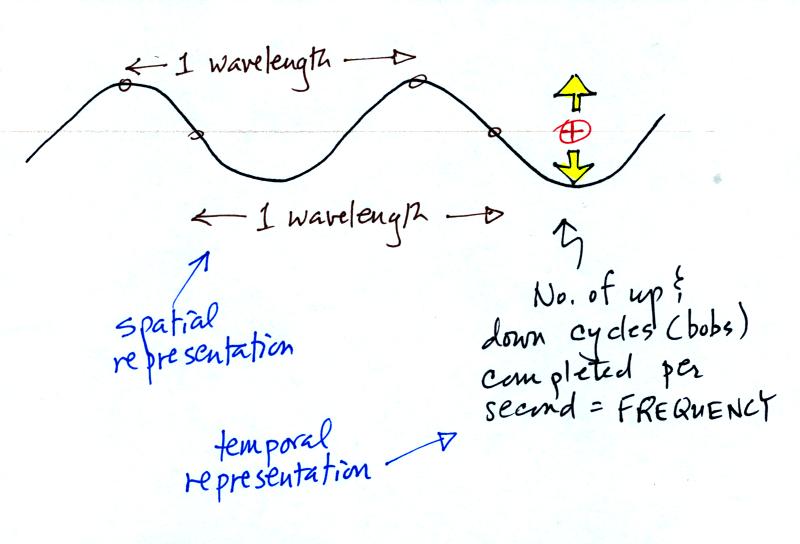
Or you can
describe the radiation temporally
using the frequency of oscillation
(number of up and down cycles completed by an oscillating charge per
second). By temporally we mean you at one particular point for a
period of time.

















