These is a little more
detailed explanation than was given in class.
Some common acids are listed below. In solution the acid
molecules dissociate (split). The presence of H+ ions
is what
makes these materials acids.

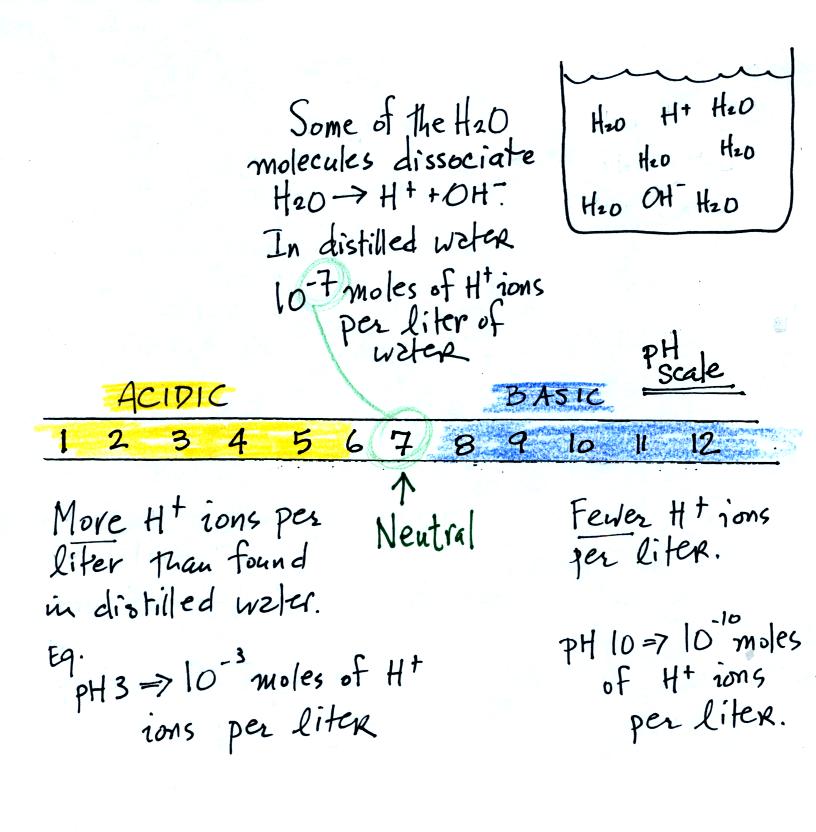
Actually
for a solution to be acidic it must have an H+ ion
concentration that
is greater than the H+ ion concentration found in distilled
water. The H+ ion concentration in pure water is 10-7
moles of H+
ions per liter of water. We often use the pH scale to measure
acid concentration. An H+ ion concentration of 10-7
moles/liter
corresponds to pH 7.
A basic solution will have an H+ ion concentration that is
lower than
found in pure water.
Now we can
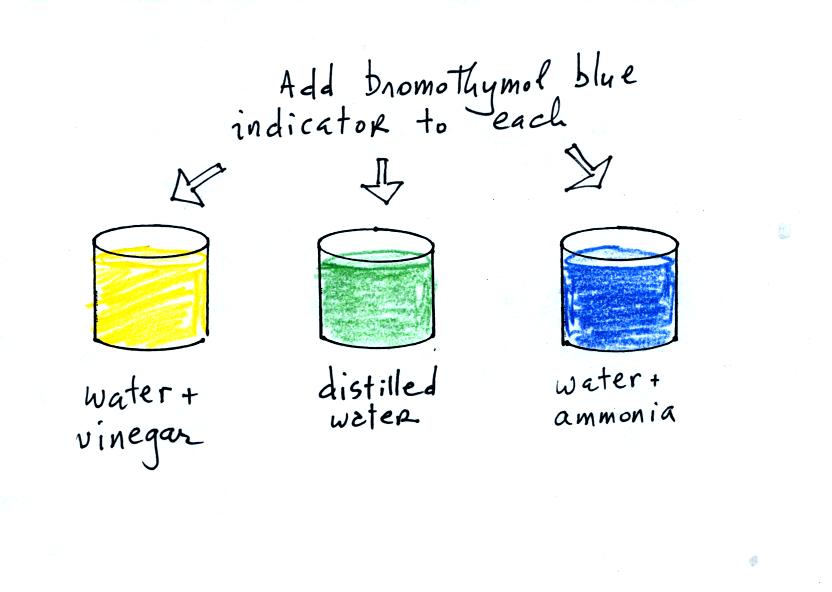
proceed to the demonstration. We will start with three 1000 mL
beakers. They have all been filled with distilled water.
Some vinegar (contains acetic acid) has been added to the left beaker.
Some ammonia (a base) has been added to the right beaker.
Then we add some bromothymol blue color indicator solution to all three
beakers. Bromothymol blue has the amazing property of changing
color depending on whether it is mixed with an acid or a base.

We add some Tucson tap water to a large 2000 mL beaker.
Tucson tap water is slightly basic. So it turns blue when we add
some bromothymol blue to it. A few small pieces of dry ice are
put into a flask. We close the flask with a stopper. The
end of a piece of tubing connected to the flask is immersed in the tap
water.
Dry ice sublimates. It turns directly from solid to ice (ordinary
ice melts and turns from solid to liquid). The gaseous CO2
is
invisible but you can tell it is there because of the bubbles in the
tap water. Some of the CO2 dissolves as it bubbles
through the
water and slowly turns the water acidic. You can tell that this
is occurring because the bromothymol blue indicator turns from deep
blue to green and eventually to yellow.





