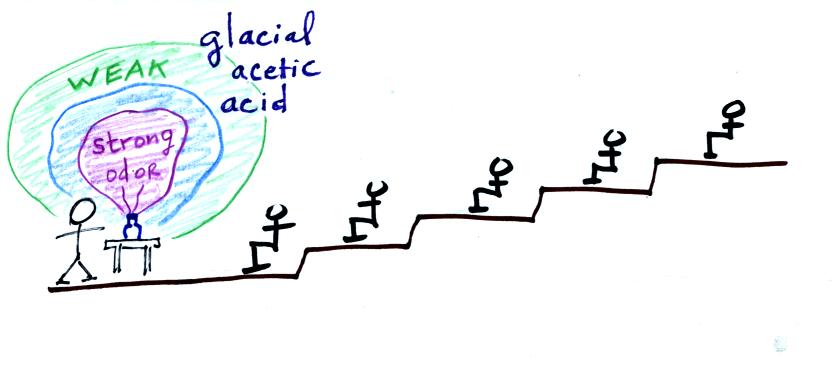
The idea was that as
the smell of the curry got into the air,
small random wind motions in the room would being to move the smell
toward the back of the room. Unfortunately the smell didn't get
very far. Maybe I'll use incense next semester.

If acetic acid had been used, the
instructor and perhaps the front row
of students would have been in a little bit of trouble by this point.
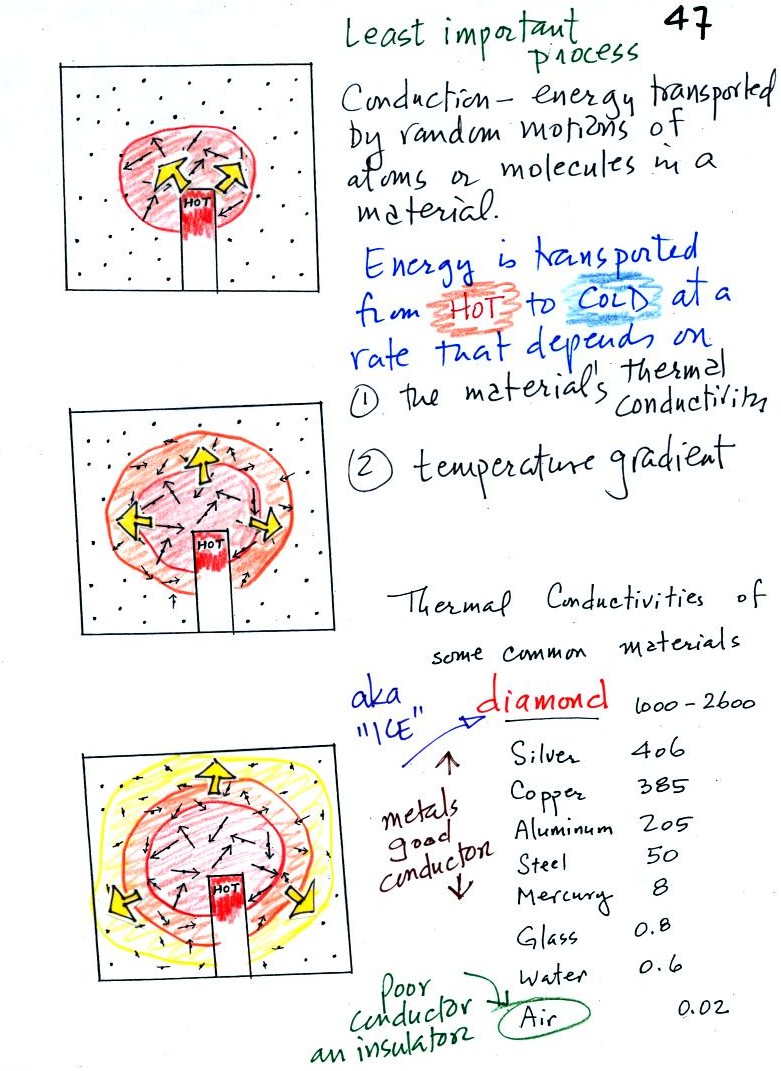
Because
air has such a low thermal conductivity it is often used as an
insulator. It is important, however, to keep the air trapped in
small pockets or small volumes so that it isn't able to move and
transport energy by convection (we'll look at convection
shortly). Here are some examples of
insulators that use air:
Small bubbles of air trapped in foam
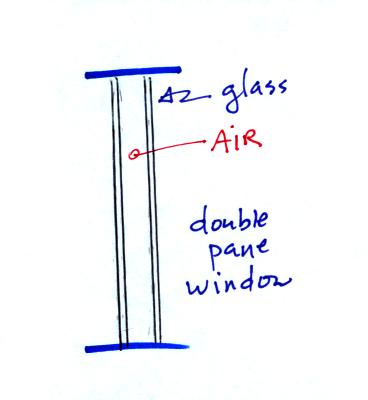 Thin insulating layer of air in a double
pane window
Thin insulating layer of air in a double
pane window
 Hollow fibers (Hollofil) filled with air used in sleeping bags and
winter coats. Goose down works in a similar way
Hollow fibers (Hollofil) filled with air used in sleeping bags and
winter coats. Goose down works in a similar way
Convection
was the next energy transport process we had a look at. Rather
than moving about randomly, the atoms or molecules move as a
group. Convection works in liquids and gases but not solids.

This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly. If I had put a
small fan behind the curry powder it would probably have spread the
smell further out into the classroom.
A thin layer of air at Point 1 in
the figure above (lower
left) is
heated by conduction. Then because hot air is also
low density air, it actually isn't necessary to blow on the hot object,
the
warm air will rise by itself (Point 3). Energy is being
transported away
from the hot object into the cooler surrounding air. This is
called free convection and
represents another way of causing rising air motions in the atmosphere
(rising air motions are important because rising air expands as it
moves into lower pressure surroundings and cools. If the air is
moist, clouds can form). Cooler air moves in to take the place of
the rising air at Point 4 and the process repeats itself.
The example at upper right is also free convection. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the warm surroundings to the colder object.
In both examples of free convection energy is being transported from
hot toward cold.
We've never really answered the question of why warm air rises and cold
air sinks. We did that next. This is covered on p. 53 in
the photocopied ClassNotes.
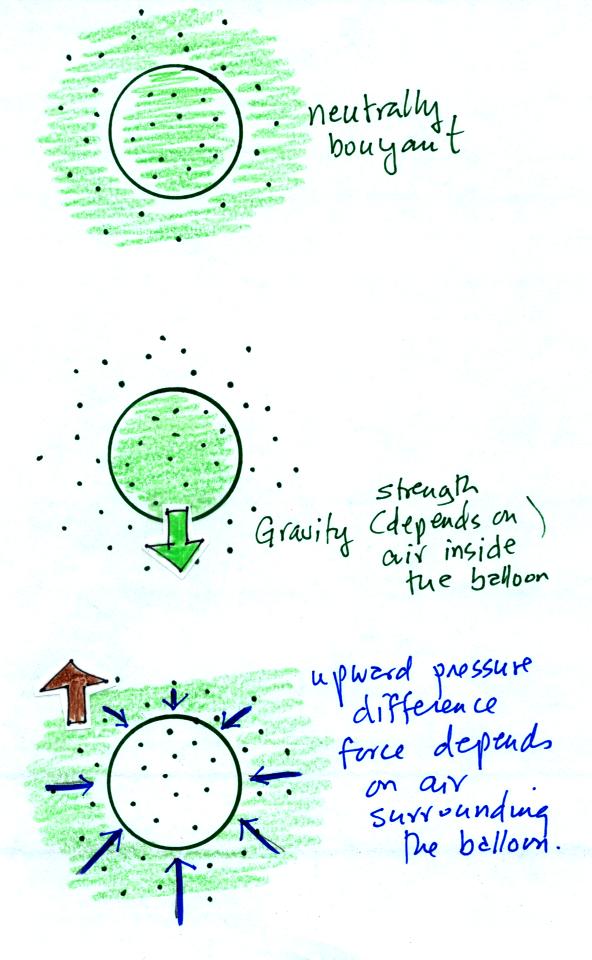
The air parcel shown in the top figure below has
the same
temperature, pressure, and density as the air around it. Under
these conditions there's no reason for the air to rise or sink, the
parcel
will remain stationary, it is neutrally bouyant.
Air has mass weight, so gravity is pulling downward on the balloon of
air (middle picture). The strength of the gravity pull (the
weight) depends on the amount of air inside the balloon.
There must be an upward force of the same strength in order for the
balloon to remain stationary. The origin of this force is the
pressure of the air surrounding the balloon. Because air pressure
decreases with increasing altitude, the forces at the top of the
balloon (pushing down) are a little weaker than the forces at the
bottom (pushing up). The net result is an upward pointing
pressure difference force. The strength of this force is
determined by the air surrounding the balloon.
Now we compare the
forces on the neutrally bouyant balloon (the green one at left) with
balloons that are filled with warm low density and cold high density
air.
If the balloon is filled with warm, low
density air the gravity force
will weaken (there is less air in the balloon so it weighs less). The
upward pressure difference force (which depends on the
surrounding air) will not change. The upward force will be
stronger than the downward force and the balloon will rise.
Conversely if a balloon is filled with cold high density air, the
balloon gets heavier. The upward pressure difference force
doesn't change. The net force is now downward and the balloon
will sink.
To demonstrate free convection we modified the Charles Law
demonstration that we did a week or two ago. We used
balloons filled with hydrogen instead of air (see bottom of p. 54 in
the photocopied Class
Notes). Hydrogen is less dense than air even when the
hydrogen has the same temperature as the surrounding air. A
hhydrogen-filled balloon doesn't need to warmed up in order to rise.
We dunked the helium-filled balloon
in some liquid nitrogen to cool
it
and to cause the density of the helium to increase. When
removed
from the liquid nitrogen the balloon didn't rise, the gas inside was
denser than the surrounding air (the purple and blue balloons in the
figure above). As the balloon warms and expands
its density decreases. The balloon at some point has the same
density as the air around it (green above) and is neutrally
bouyant. Eventually the balloon becomes less dense that the
surrounding air (yellow) and floats up to the ceiling.
Now some
practical applications of what we have learned about conductive and
convective energy transport. Energy transport really does show up
in a lot more everydat real life situations than you might expect.
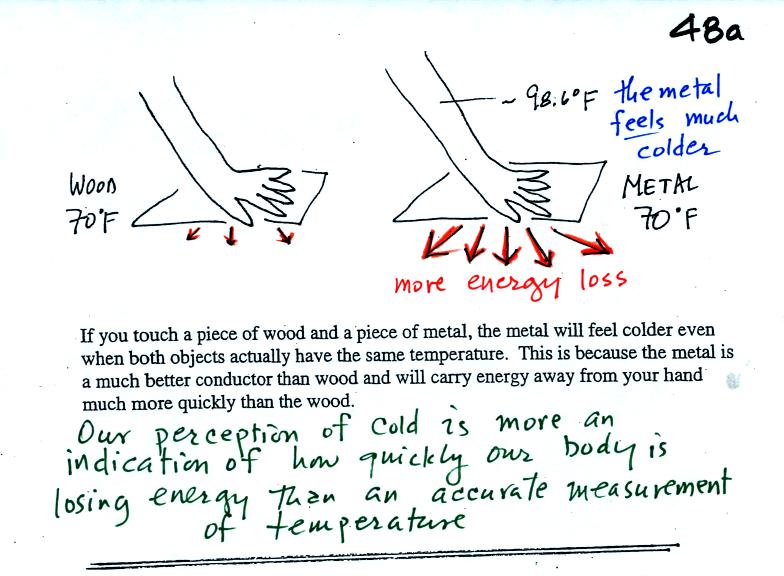
Note first of all there is a temperature difference between
your hand and a 70o F object. Energy will flow from your warm
hand to the colder object. Metals are better conductors than
wood. If you touch a
piece of
70 F metal it will feel much colder than a piece of 70 F wood, even
though they both have the same temperature. You'll be able to
check this out in class on Friday.
A
piece
of 70 F diamond would feel even colder because it is an even better
conductor
than metal. Something that feels cold may not be as
cold as it seems. Our perception of cold is more an
indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
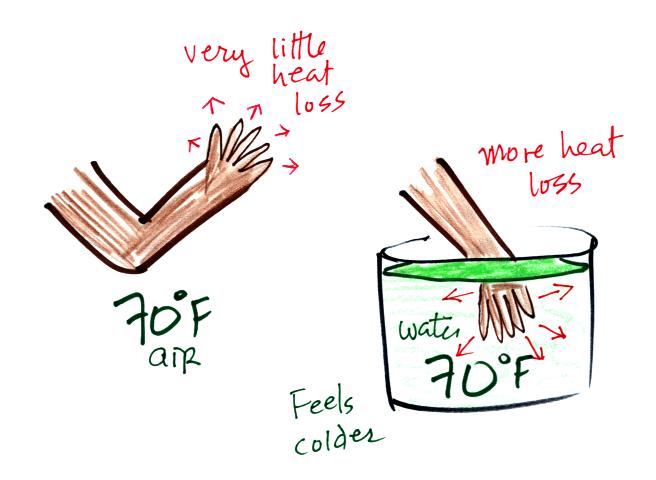
Here's a similar situation. This
picture wasn't shown in class.
You can stand outside in 70 F without any problem. You probably
wouldn't feel cold. But if you jump into 70 F pool water you will
feel cold, at least until you "get used" to the water temperature (your
body might reduce blood flow to your extremeties and skin to try to
reduce energy loss)
Air is a poor conductor. If you out in
40 F
weather you will feel colder largely because there is a larger
temperature difference between you and your surroundings.

If you stick your hand
into a bucket of 40 F water, it will feel very cold (your hand will
actually soon begin to hurt). Water is a much better conductor
than air. Energy flows much more rapidly from your hand into the
cold water.
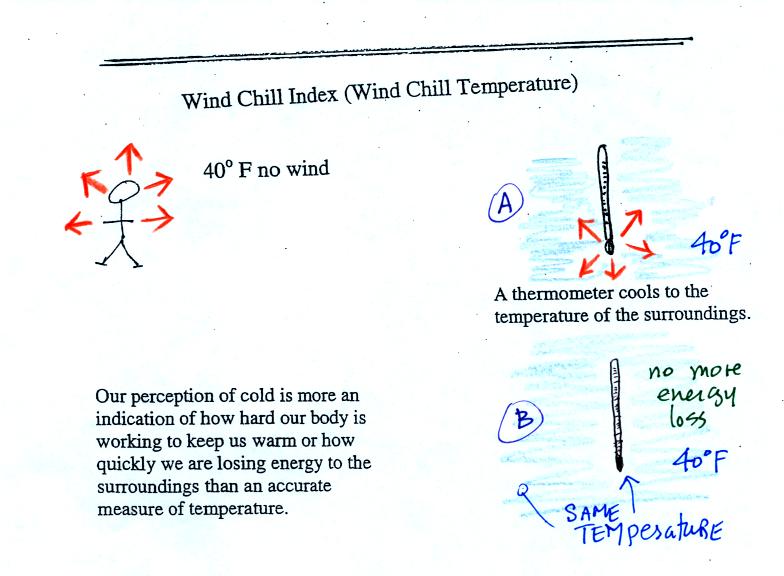
Now we're
in a perfect position to understand wind chill.
If you go outside on a 40 F day (calm winds) you will
feel
cool; your
body is losing energy to the colder surroundings (by conduction
mainly). Your body works hard to keep its core temperature around
98.6 F. A thermometer
behaves differently. It actually cools to the temperature of the
surroundings. Once it reaches 40 F it won't lose any additional
energy.

Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a calm 28
F day. Your body is losing energy at the same rate in both
cases. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F.
The thermometer will again cool to the
temperature of its surroundings, it will just cool more quickly on a
windy day. Once the thermometer reaches 40 F there won't be any
additional energy flow. The
thermometer would measure 40 F on both the calm and the windy day.
Standing outside on a 40 F day is usually not a life threatening
situation. Falling into 40 F water is.
 Energy will be conducted away from your body more quickly than
your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be sure not to confuse hypothermia with hyperthermia which
can bring on
heatstroke and which is also a serious outdoors risk in S.
Arizona.
Energy will be conducted away from your body more quickly than
your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be sure not to confuse hypothermia with hyperthermia which
can bring on
heatstroke and which is also a serious outdoors risk in S.
Arizona.