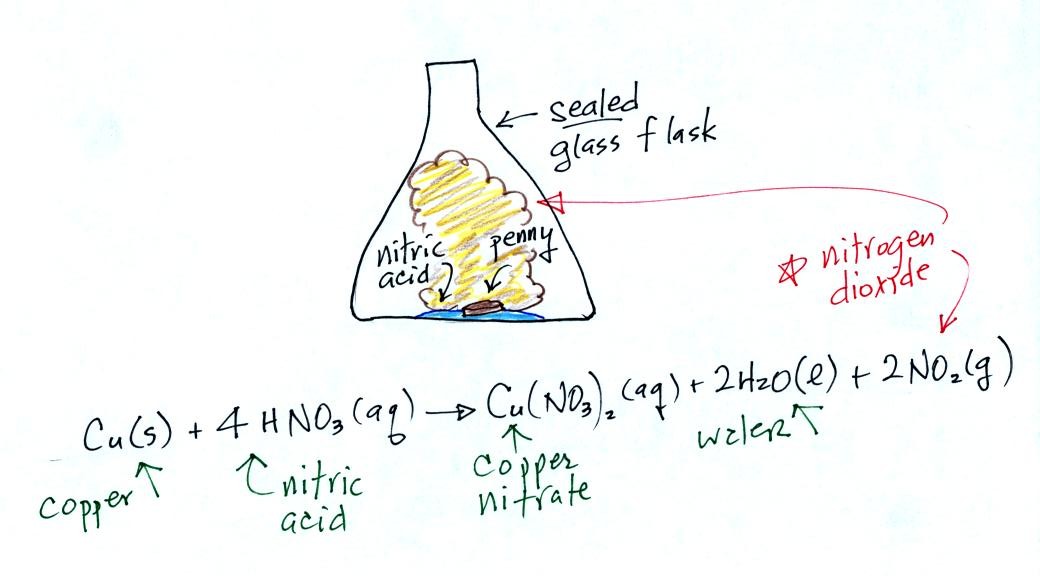
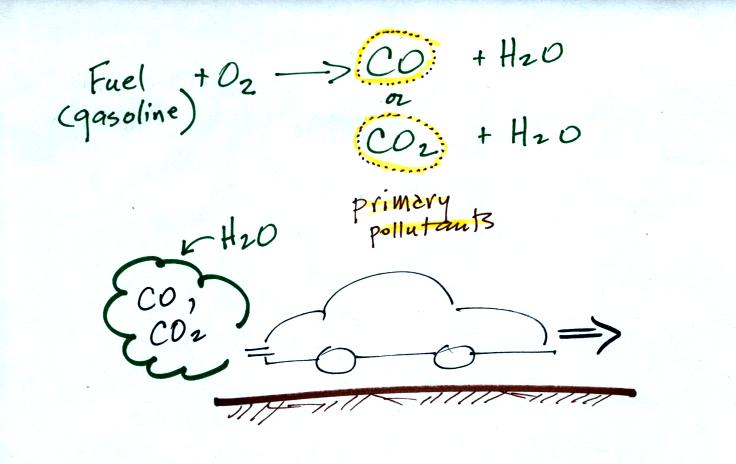
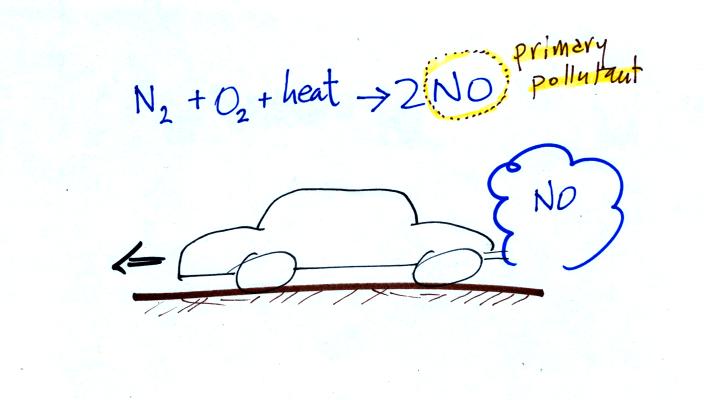
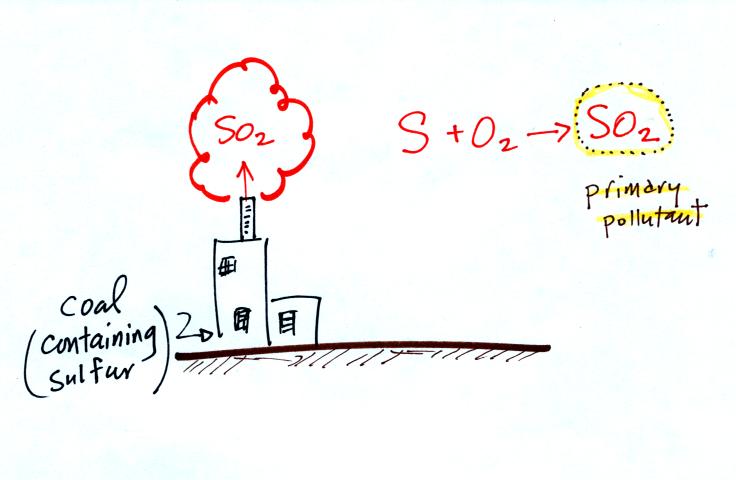
Thunderstorms contain strong up
(updraft) and down (downdraft) air motions. Thunderstorms are a
sure indication of unstable atmospheric conditions. When the
downdraft winds hit the ground they spread out horizontally.
These surface winds can sometimes reach 100 MPH, stronger than many
tornadoes. An unusually strong and narrow thunderstorm downdraft
is called a microburst.
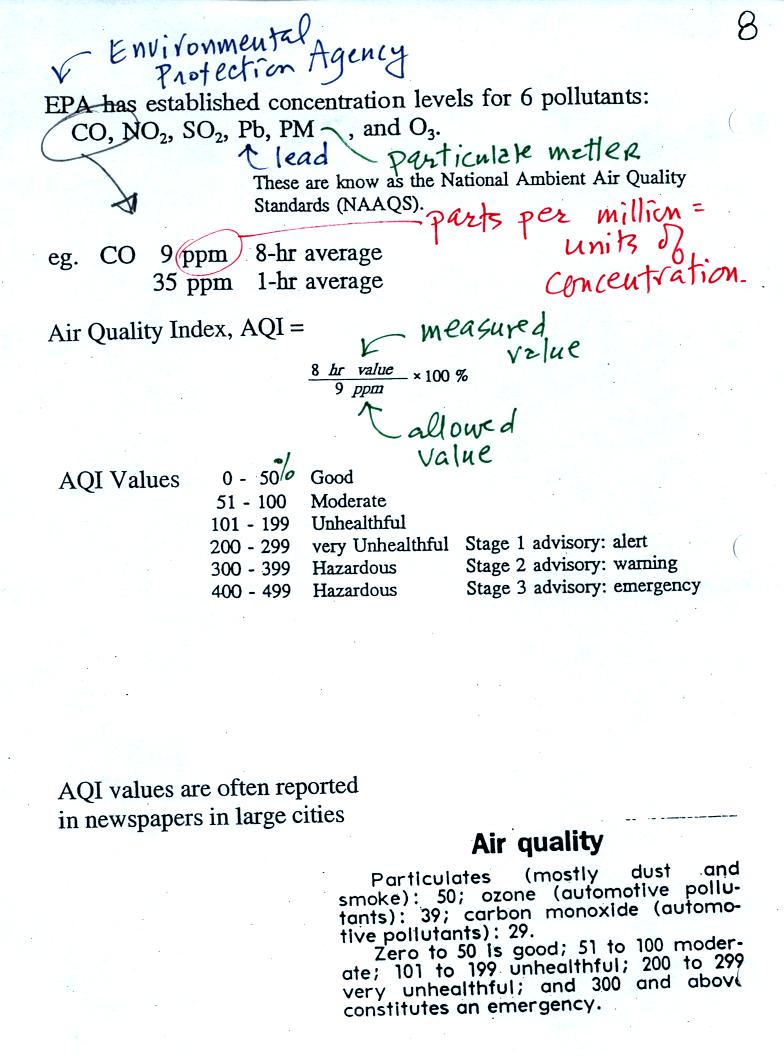
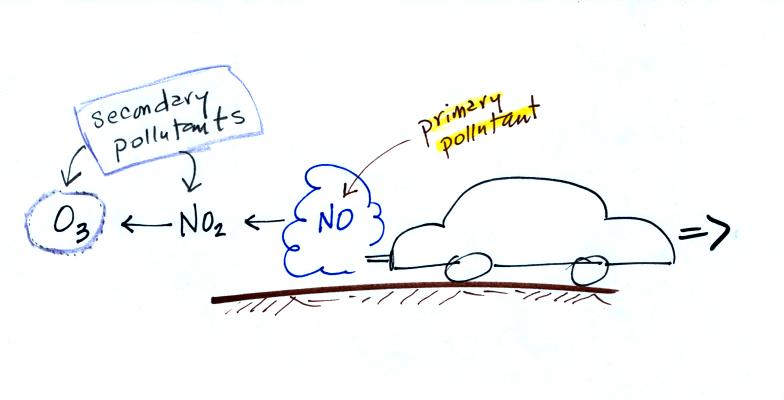
Six main air pollutants are listed at the top of this page.
Concentrations of some or all of these pollutants are monitored daily
in
many
cities. The atmospheric concentration of lead has decreased
significantly since the introduction of unleaded gasoline. PM
stands for particulate matter. These small particles are
invisible, remain suspended in the air, and may be made of harmful
materials.
CO, O3 and particulate matter are the pollutants of
most
concern in
Tucson and pollutant concentrations are reported in the newspaper or on
television using the Air Quality Index (formerly the pollutant
standards index). This is basically the measured value divided by
the allowed value multiplied by 100%. For carbon monoxide
concentrations up to 35 ppm (parts per million) for a 1 hour period and
9 ppm for an 8 hour period are allowed. Current Air Quality Index values for
Tucson are available online.
Yearly changes in the AQI values for ozone and carbon
monoxide measured in Tucson in 1993
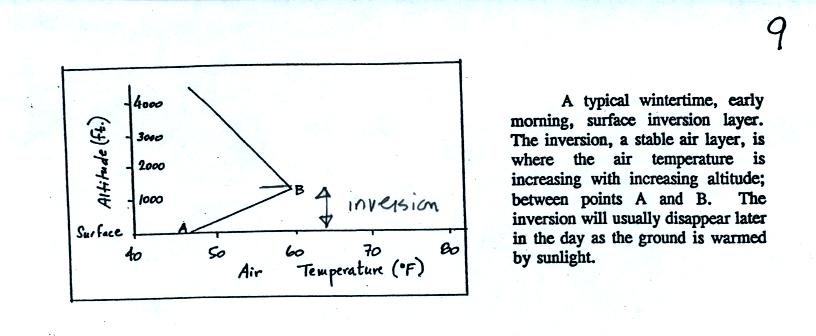
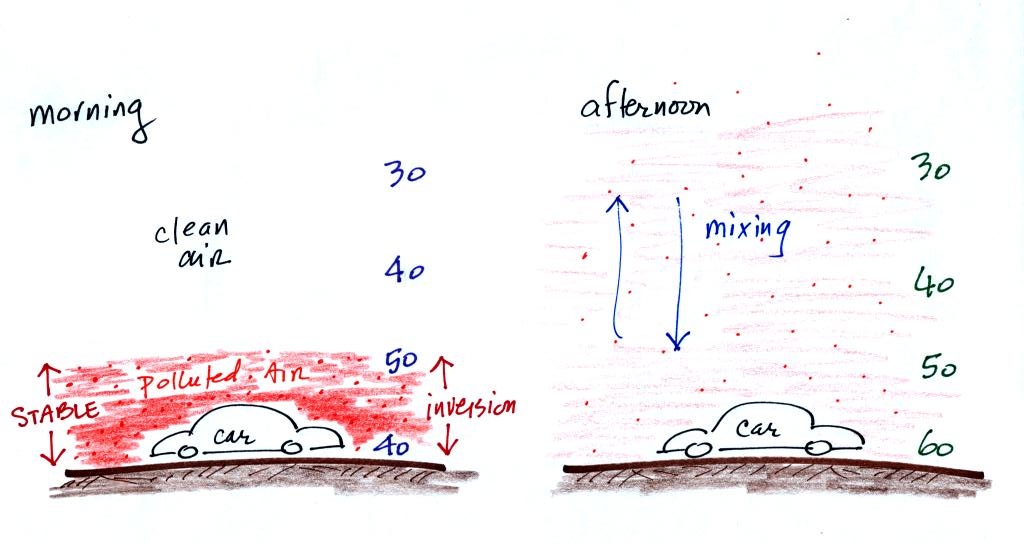
are plotted at the bottom of p.9 in the photocopied Classnotes. This figure wasn't shown in class.
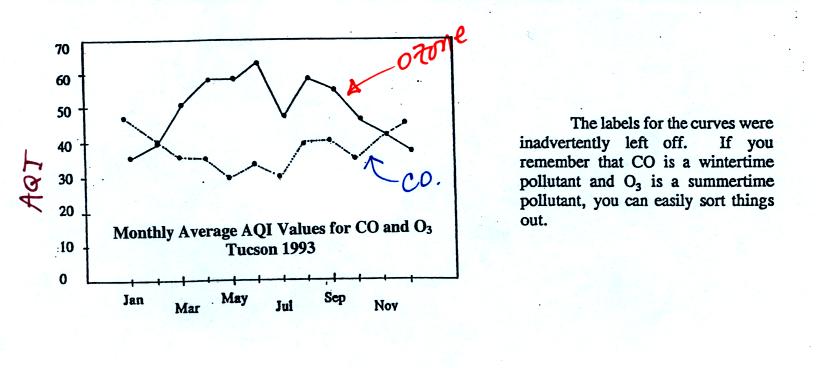
There are a couple of things to
note in this figure. First the highest AQI values for carbon
monoxide are observed in the winter. CO is a winter morning
pollutant. The highest ozone AQI values are observed in the
summer. Ozone, it turns out, is a summer afternoon pollutant
(we'll learn why next week). Also ozone AQI values almost reach
70 in the summer. There are many people that think this is high
enough to present a risk to people with existing lung disease.
So are we have been talking about carbon monoxide found in
the atmosphere. Carbon monoxide is also a serious
hazard indoors where is can build to much higher levels than would ever
be found outdoors. You may remember having heard
about an incident at the beginning of the school year in 2007. Carbon
monoxide
from a malfunctioning hot water heater sickened 23 Virginia Tech
students in an apartment complex. The CO concentration is
thought to have reached 400 ppm. You can get an idea of what
kinds of health effects concentrations this high could cause from the
figure below (from p. 9 in the photocopied Classnotes). This figure wasn't shown or discusses
in class.
Follow the 400 ppm line (shaded orange) from left to right.
At
exposure times less than 1 hour you should experience no
symptoms. Beginning at 1 hour you might experience headache,
fatique, and dizziness. Exposures of a few hours will produce
throbbing headache, nausea, convulsions, and collapse. The 400
ppm trace level approaches the level where CO would cause coma and
death. At Virginia Tech several students were found unconscious
and one or two had stopped breathing.
Carbon monoxide
alarms are relatively inexpensive and readily available at any hardware
store. They will monitor CO concentrations indoors and warn you
when
concentrations reach hazardous levels. Indoors CO is
produced by gas furnaces and water heaters that are
either operating improperly or aren't being adequately vented
to the outdoors. A few hundred people are killed indoors by
carbon
monoxide every
year in the United States. You can learn
more about carbon monoxide hazards and risk prevention at the Consumer Product
Safety Commission web page.
We'll wait
until next Tuesday to discuss p. 10 in the photocopied
ClassNotes. It involves bicycling, one of my favorite topics.