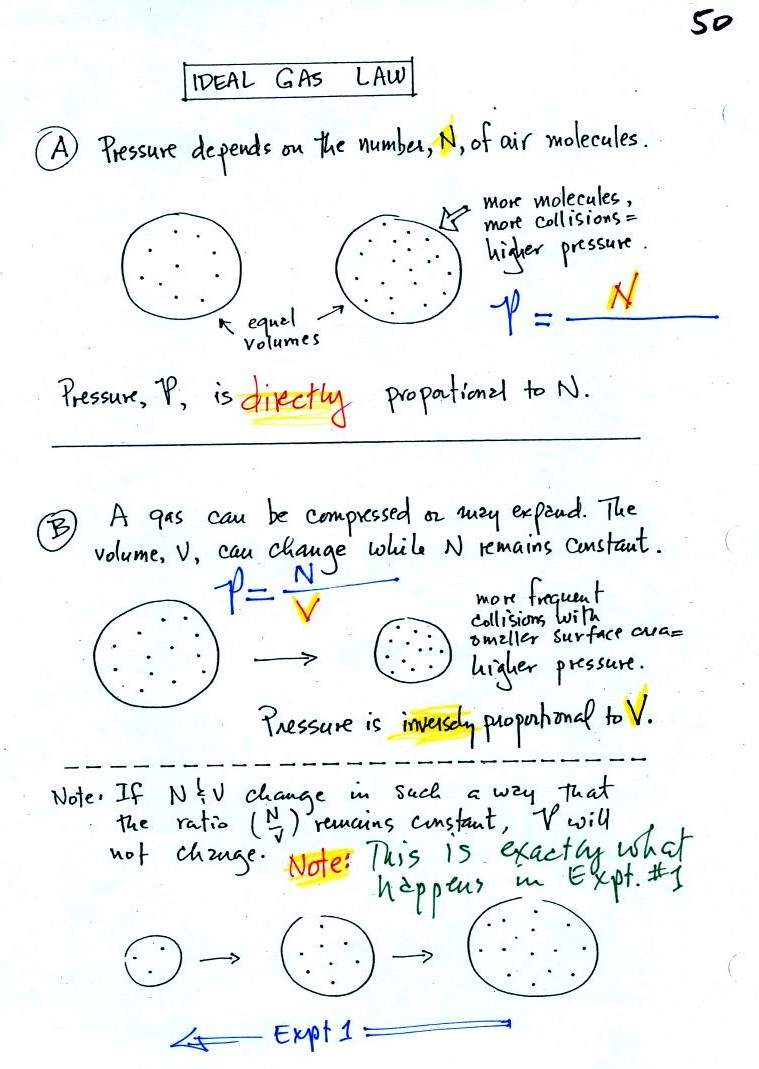
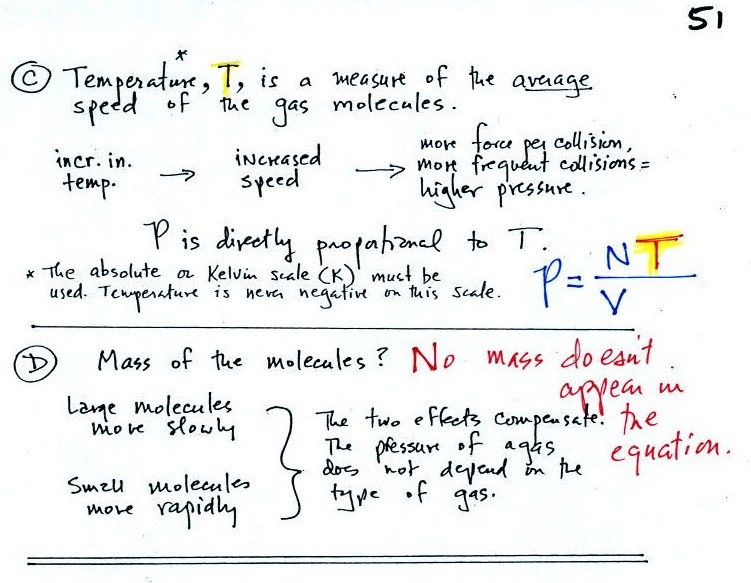
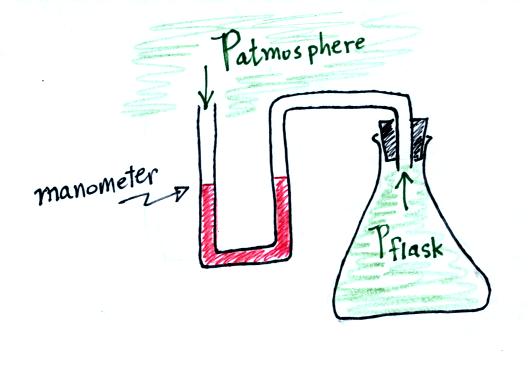
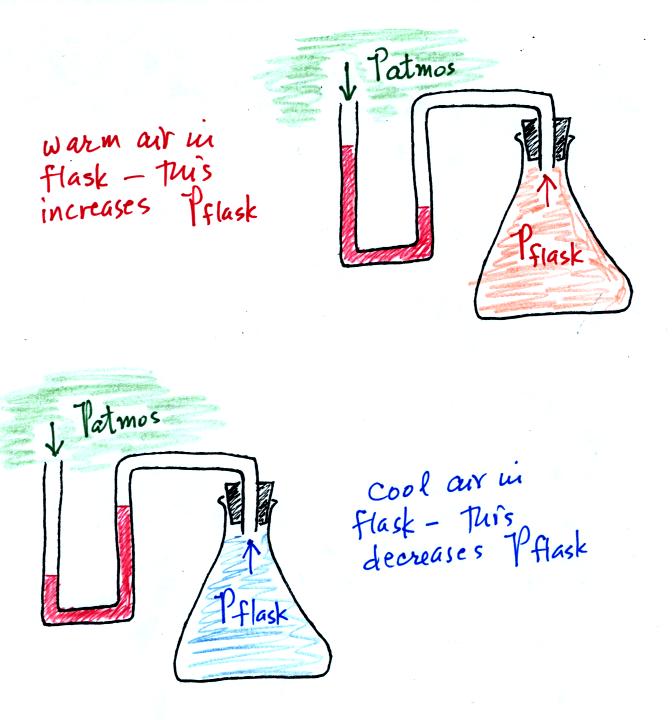
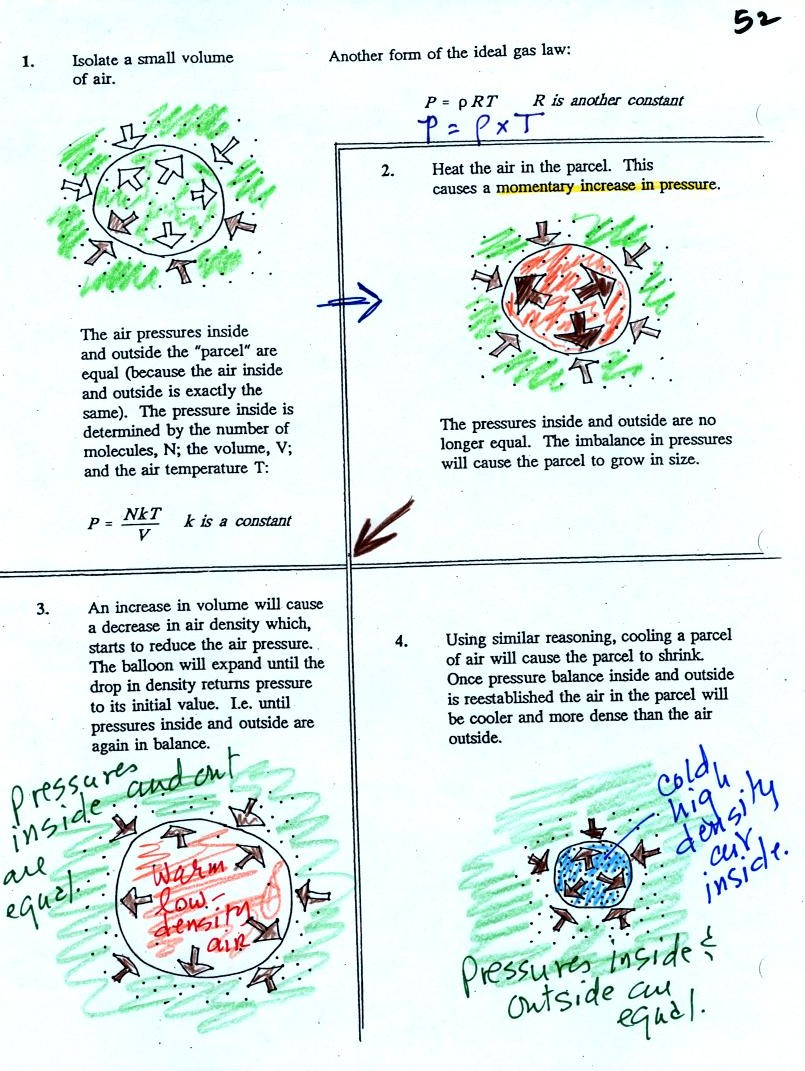
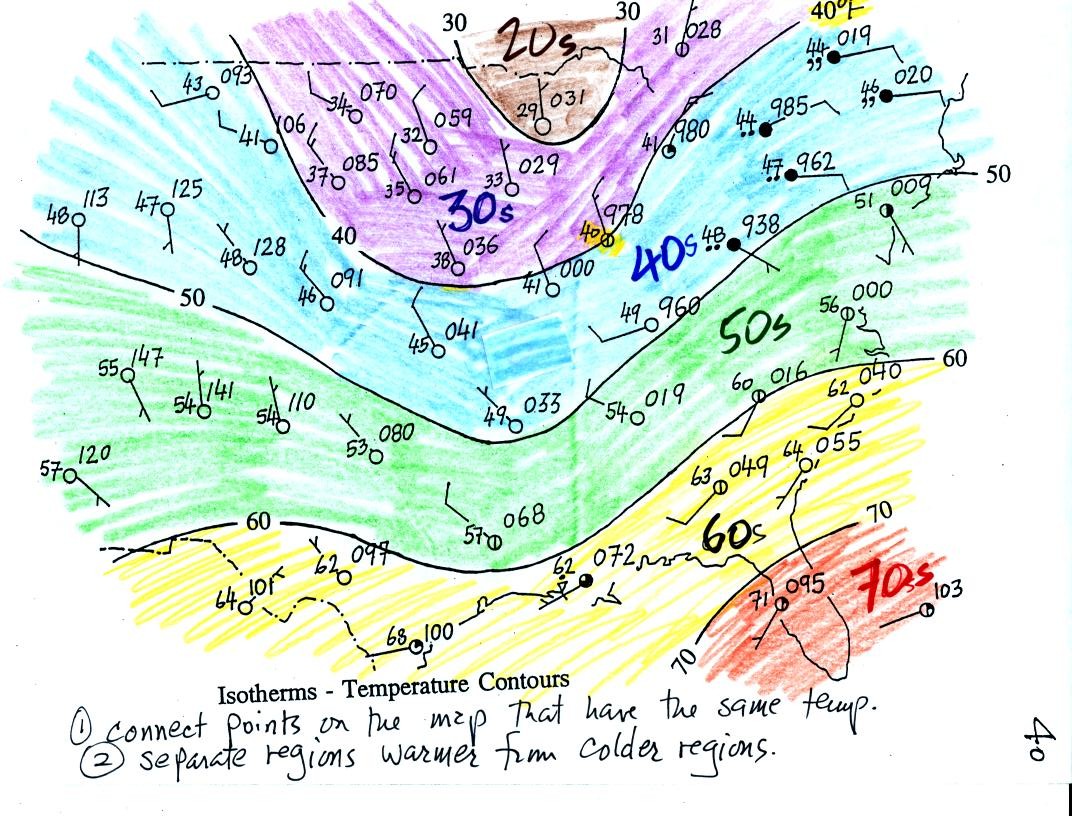
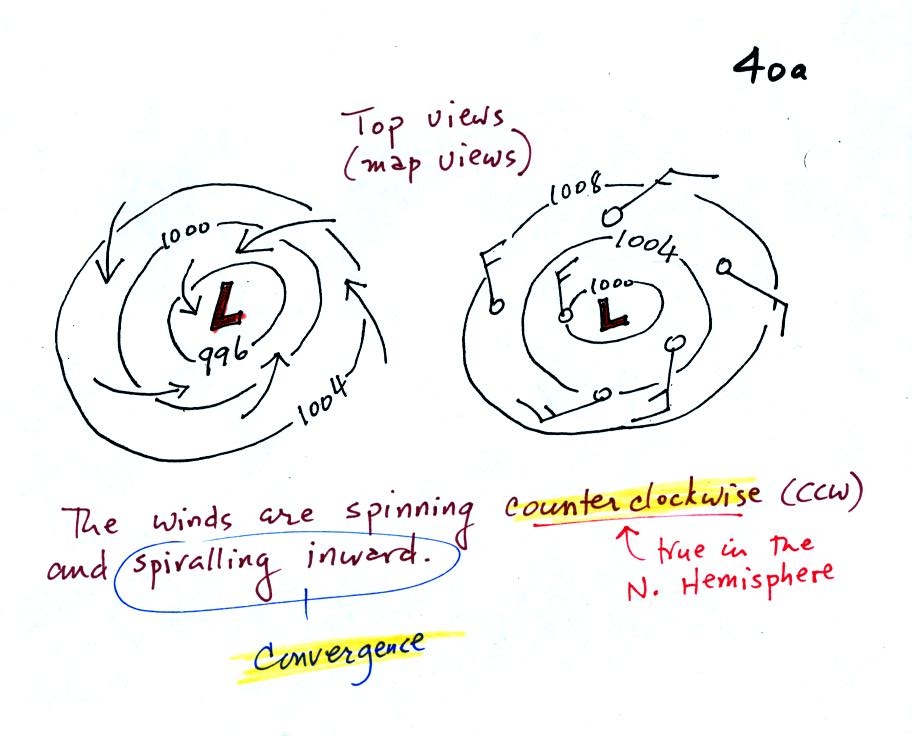
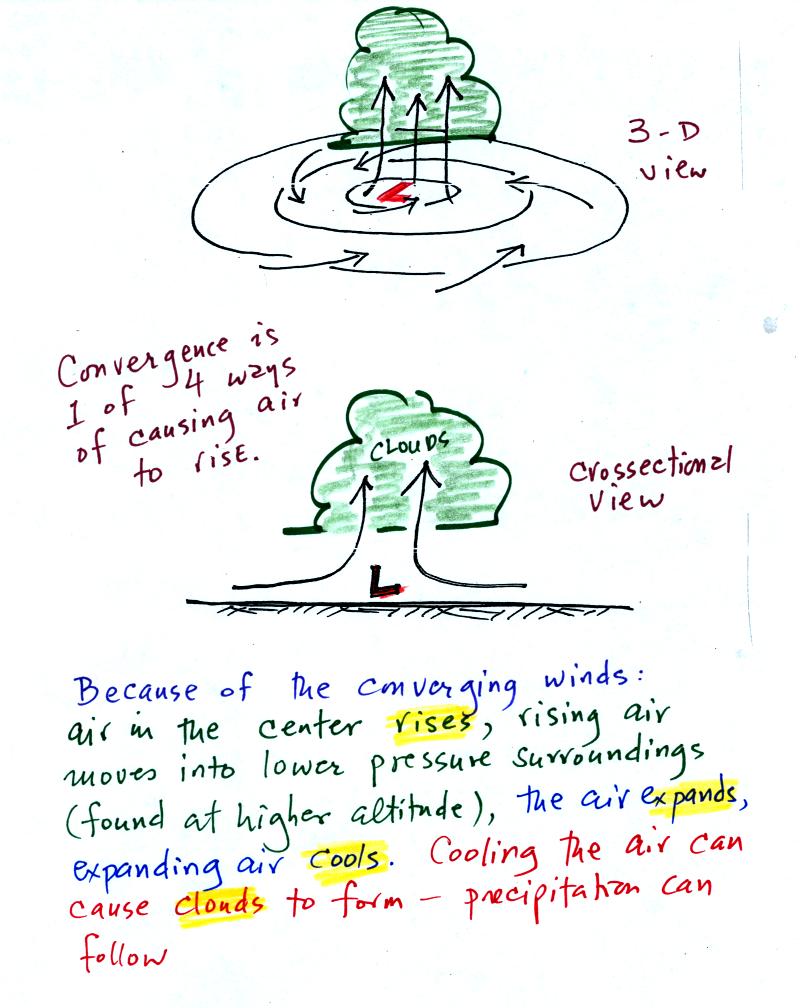
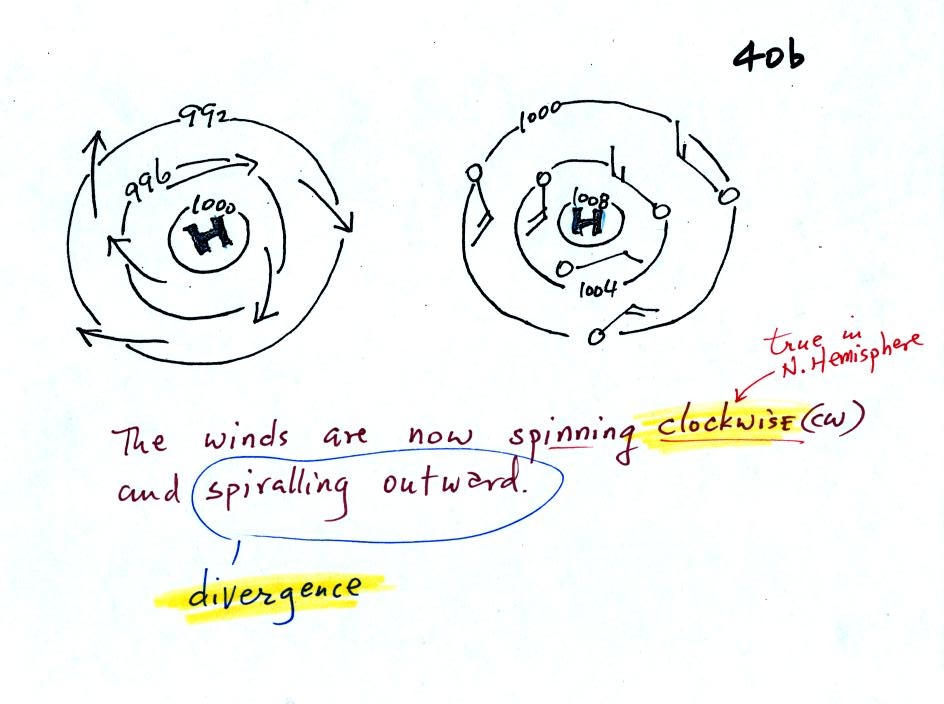
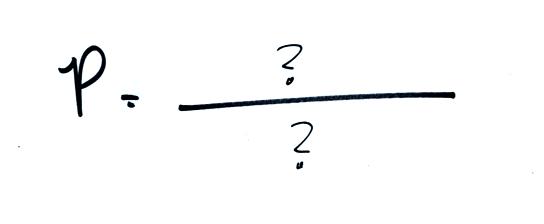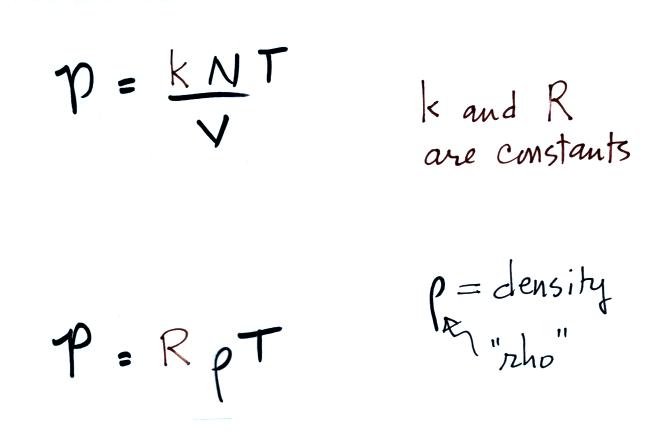Read through the explanation on p.
52 in the photocopied
Classnotes.
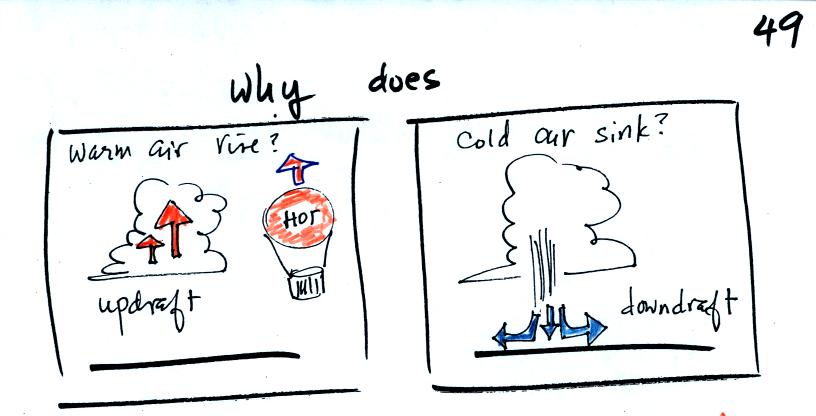
These two associations:
(i) warm air = low
density air
(ii) cold air = high density air
are important and will come up a lot during the remainder of the
semester.
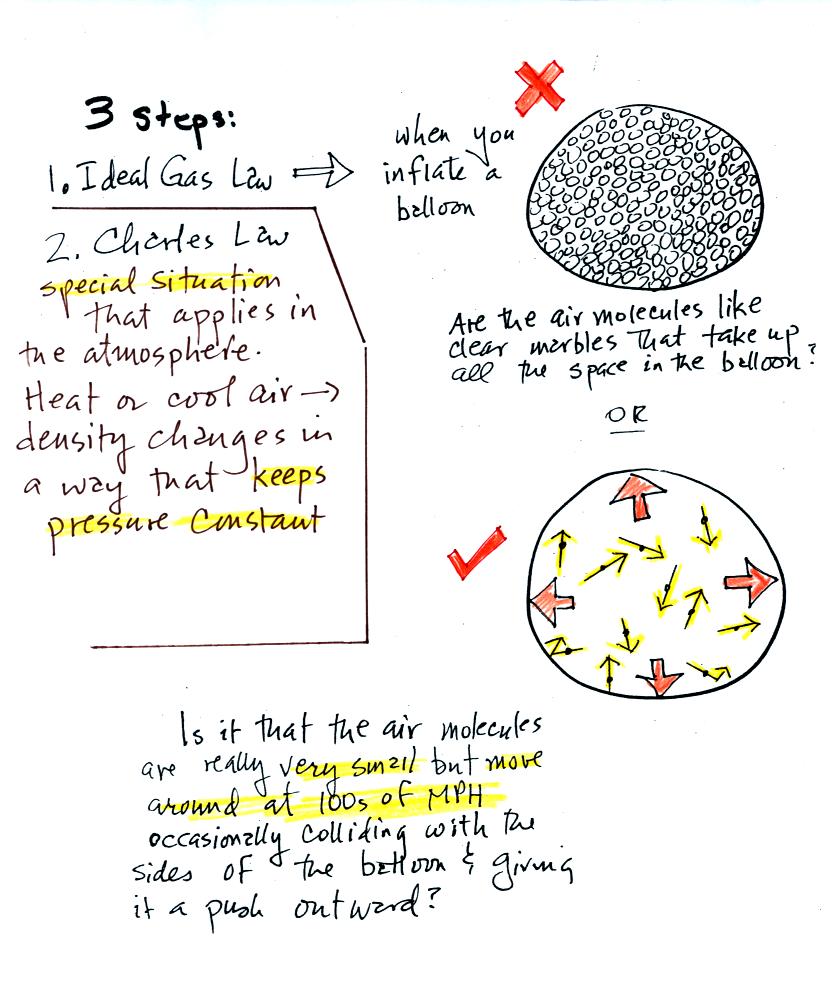
Click here if you would like a little
more detailed, more step-by-step,
explanation of Charles Law. Otherwise proceed on to the Charles'
Law demonstration that we did in class.
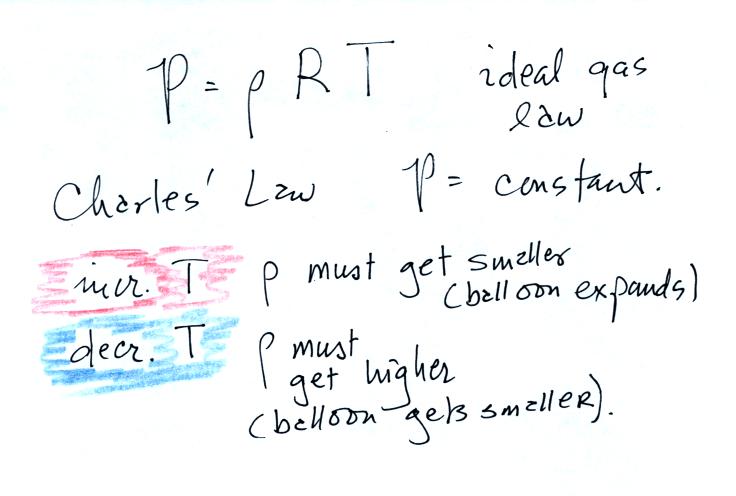
Charles Law can be demonstrated by dipping a balloon in
liquid
nitrogen. You'll find an explanation on the top of p. 54 in the
photocopied ClassNotes.
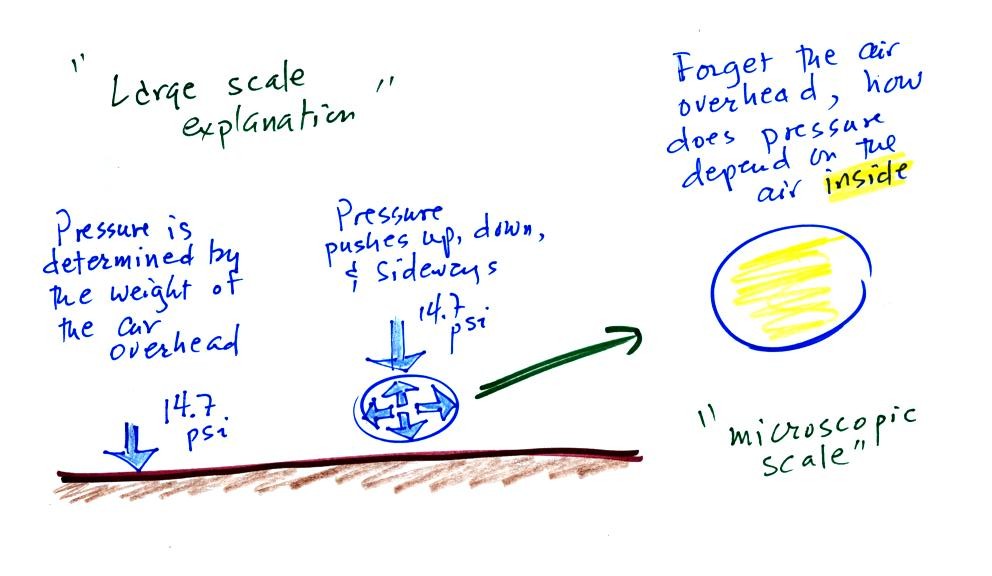
The balloon had shrunk down to practically no volume when
pulled from the liquid nitrogen. It was filled with cold high
density air. As
the balloon warmed the balloon expanded and the density of the air
inside
the balloon decreased. The volume and temperature kept changing
in a way that kept pressure constant.
Here's a summary