Monday Aug. 31, 2009
click here to download today's notes
in a more printer friendly format
A few more sets of Experiment #1 materials
were handed out in class today. I'll bring the remaining kits of
materials to class one more time on Wednesday.
Two songs were played before class today: "Punish the Monkey"
and "The Trawlerman Song" from Mark Knopfler. Both songs had a
kind of
surf guitar sound to them which seemed fitting given the unveiling of
the
Tiki last Saturday night on 4th Avenue.
Tiki unvieled!
From the Arizona Daily Star Monday Aug. 31
Taken 08/29/09 by Joel Smith on 4th Avenue at the Tiki
unveiling.
Just want to take this moment to say that there were well
over 1,000 people
in +100 degree heat and a good time was had by all as
far as I could see.
We began
class by quickly reviewing material on the Air Quality Index (AQI)
health effects of carbon monoxide that was stuck into the Friday Aug.
28 notes.
I have a bad habit of "beating some concepts to
death."
Here's an example. This rather
busy and confusing picture just illustrates how small changes in how
air temperature changes with increasing altitude can determine whether
the atmosphere will be stable or unstable. Just for
the
purposes of illustration imagine riding a bicycle north from Swan and
River Rd up the hill to Swan and Sunrise (fhe figure shows an elevation
change of 1000 ft, it is actually quite a bit less than that).
The figure below is a little neater version of what was drawn in class.

At far left the air temperature goes from 47o F to 41o
F, a drop of 6o
F. This is a
fairly
rapid rate of decrease with increasing altitude and would make the
atmosphere
absolutely unstable.
The atmosphere wouldn't remain this
way. Air at the ground would rise, air higher up would sink, and
the
temperature profile would change. In some ways it would be like
trying to pour vinegar on top of oil in a glass. The lower
density oil would rise because it would "want" to float on top of the
higher density vinegar.
The next picture shows air temperature decreasing a little more slowly
with increasing altitude. This small change makes the atmosphere
conditionally unstable
(we won't go into what the conditions might
be). The
atmosphere is frequently in this state.
The atmosphere cools only 2o F in the next picture.
This creates
an absolutely stable
atmosphere. Air at the ground will remain at
the ground and won't rise and mix with air higher up. Compare
this with the glass containing vinegar and a layer of oil on top.
The two layers won't mix.
Air temperature in the last figure actually increases with increasing
altitude. This is a temperature
inversion and is very common on
winter mornings. The atmosphere is extremely stable under
these conditions.
Temperature inversions are something you can check out for yourself:
head north on Swan
Rd. on your bicycle early some winter morning. You will pass
through
some pretty cold air as
you cross the Rillito River. By the time you get to Sunrise, the
air can be 10 to 15 degrees warmer and will seem balmy compared to the
cold
air at the bottom of the hill. If you're up for a real
hill-climbing challenge
continue north on Swan past Skyline. You'll find a short but very
steep section of road at the far north end of Swan.
Next we moved on to the 2nd air pollutant that we will be discussing -
sulfur dioxide. Here's some basic information from the left hand
of p. 11 in the photocopied ClassNotes.
Sulfur dioxide is produced by the combustion of sulfur
containing
fuels such as coal. Combustion of fuel also produces carbon
dioxide and carbon monoxide. People probably first became aware
of sulfur dioxide because it has an unpleasant smell. Carbon
dioxide and carbon monoxide are odorless. That is why sulfur
dioxide was the first pollutant people became aware of.
Volcanoes are a natural source of sulfur dioxide.
The
Great
London smog is still one of the two or three deadliest air pollution
events in
history.
Because the atmosphere was stable, SO2
emitted into air
at ground level couldn't mix with cleaner air above.
The SO2
concentration was able to build to dangerous levels.
4000 people
died during this 4 or 5 day period.
As many as 8000 additional
people died in the following weeks and months.
Some
of the photographs below come from articles published in 2002 on the
50th anniversary of the event.
The sulfur dioxide didn't
kill people directly.
The SO2 aggravated
an existing
condition of some kind and hastened their
death.
The SO2 probably also made people susceptible
to bacterial
infections such as pneumonia.
This
link discusses the event and its health effects in more detail.
London type smog which contains sulfur dioxide and is most
common
during the winter is very different from photochemical or Los Angeles
type smog. Los Angeles type smog contains ozone and is most
common in the summer.
Some other air pollution disasters also involved high SO2
concentrations.
One of the deadliest events in the US occurred in
1948 in Donora, Pennsylvania.
"This eerie photograph was taken at noon on Oct.
29, 1948 in Donora, PA as deadly smog enveloped the town. 20 people
were asphyxiated and more than 7,000 became seriously ill during this
horrible event."
from: http://oceanservice.noaa.gov/education/kits/pollution/02history.html
from: http://www.eoearth.org/article/Donora,_Pennsylvania
"When Smoke Ran Like Water," a
book
about air pollution is among the books that you can check out, read,
and report on to fulfill part of the writing requirements in this class
(instead of doing an experiment report). The
author, Devra Davis, lived in Donora Pennsylvania at the time of the
1948 air pollution episode.
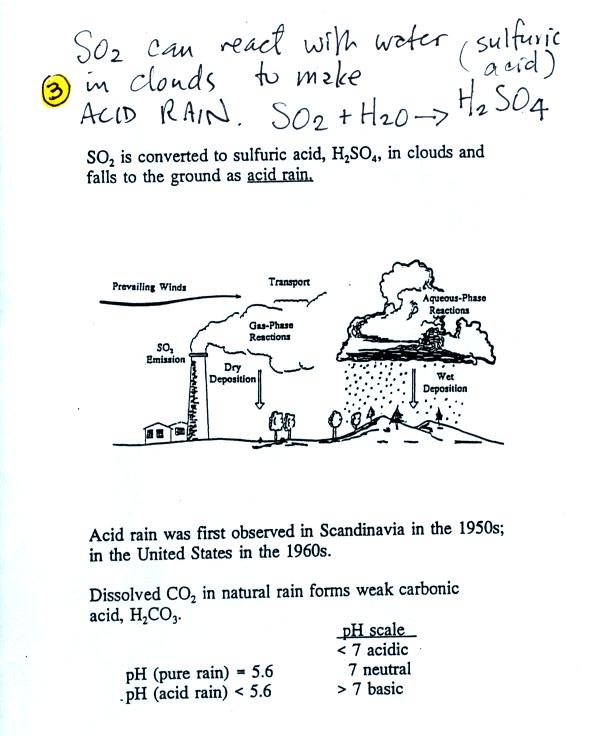
Sulfur
dioxide is one of the pollutants that can react with water in
clouds to form acid rain (some of the oxides of nitrogen can react with
water to form nitric acid). The formation and effects of acid
rain
are discussed on p. 12 in the photocopied Class Notes.
Note that clean unpolluted rain has
a pH less than 7
and is
slightly
acidic. This is because the rain contains dissolved carbon
dioxide gas. We saw how this happens in a class demonstration.
Acid rain is often a problem in regions that are
100s even 1000s of miles from the source of that sulfur dioxide that
forms the acid rain. Acid rain in Scandinavia came from
industrialized areas in other parts of Europe.
Some of the problems associated with acid rain.
Finally we
performed a sort of acid
rain demonstration. The
demonstration gives you an idea of
how gases can dissolve in water and turn the water acidic.