Wednesday Oct. 7, 2009
click here to download today's notes in
a more printer friendly format
A couple of songs from an Austin TX group that I just heard about a
week or two ago Grupo
Fantasma
Some how or another I have finally managed to read and grade all of the
1S1P reports on radon. They were returned in class today.
The Optional Assignments were collected today. You should get
those back next Monday at the latest. In the meantime here are
the answers
to the questions on the assignment.
The complete Quiz #2 Study Guide is now available in preliminary form
(mostly it is a question of how much of the material on the study guide
we will cover in class before the quiz). Quiz #2 is next
Wednesday, Oct. 14.
The Expt. #2 reports are due next Monday. Please try to return
your materials this week.
We learned a little bit about energy transport by conduction and
convection in class on Monday. We also learned that our
perception of cold is a better indicator of how quickly our body is
losing energy than an accurate measurement of temperature.
This basic knowledge puts us in a perfect position to understand the
concept of wind
chill temperature.
If you go outside on a 40 F day (calm winds) you will
feel
cool; your
body is losing energy to the colder surroundings (by conduction
mainly). Your body works hard to keep its core temperature around
98.6 F. A thermometer
behaves differently, it is supposed to cool to the temperature of the
surroundings. Once it reaches 40 F it won't lose any additional
energy.
If you go outside on a 40 F day with 30 MPH winds your
body
will lose
energy at a more rapid rate (because convection together with
conduction are transporting energy away from your body). This
higher rate of energy loss will make it feel colder
than a 40
F day
with calm winds.
Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a 28
F day without any wind. Your body is losing energy at the same
rate in both
cases. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F.
The thermometer will again cool to the
temperature of its surroundings, it will just cool more quickly on a
windy day. Once the thermometer reaches 40 F there won't be any
additional energy flow. The
thermometer would measure 40 F on both the calm and the windy day.
Standing outside on a 40 F day is not an immediate life
threatening
situation. Falling into 40 F water is.
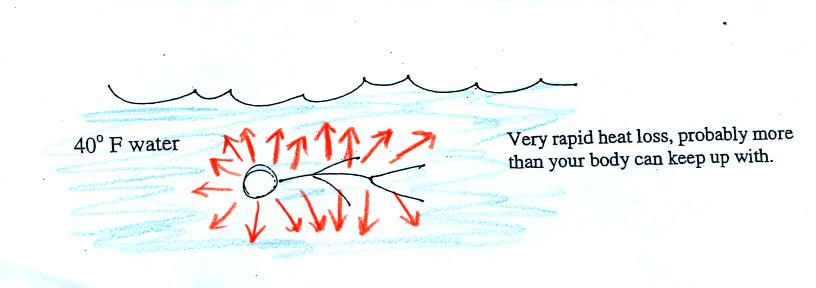
Energy will be conducted away from your body more quickly
than
your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be sure not to confuse hypothermia with hyperthermia which
can bring on
heatstroke and which is also a serious outdoors risk in S.
Arizona.
At this
point I showed a
picture from the March 2005 issue of National Geographic. A Buddhist
monk was standing in a frigid waterfall. The caption for the
photograph read "To focus the mind and increase awareness of self,
Shingon Buddhists like Souei Sakamoto practice takigyo,chanting
for hours while standing in frigid waterfalls at the Oiwasan Nissekiji
Temple in Toyama, Japan." (I can't really scan the photograph and
include it in the classnotes because of copyright laws)
A second photograph from the December 2005 issue showed a monk hanging
from a tree by his feet. The caption there read "To
see life as it truly is - that's the goal of a student in China who
strengthens mind and body under the rigorous tutelage of a Shaolin kung
fu master."
Perhaps the most amazing example of a physical and mental task is the
1000-day challenge undertaken by the "marathon
monks" of Mount Hiei, Japan.
I hope you don't mind an
occasional digression like this. I spend a lot of time
riding my bicycle
uphills. It's not really painful but can definitely be
uncomfortable. I've
noticed that you can sometimes be distracted by a thought and ride a
mile or so and completely blank out the discomfort. With some
"Buddhist monk like" training I wonder if maybe I couldn't ride uphill
more or less indefinitely and not feel any discomfort at all.
This time of the year it is often a little cool in the morning.
In another month or so it will be cold. With some mental training
I hope be to be able to blank the feeling of
cold fingers and toes. I'm not there yet but will continue to
work on it.
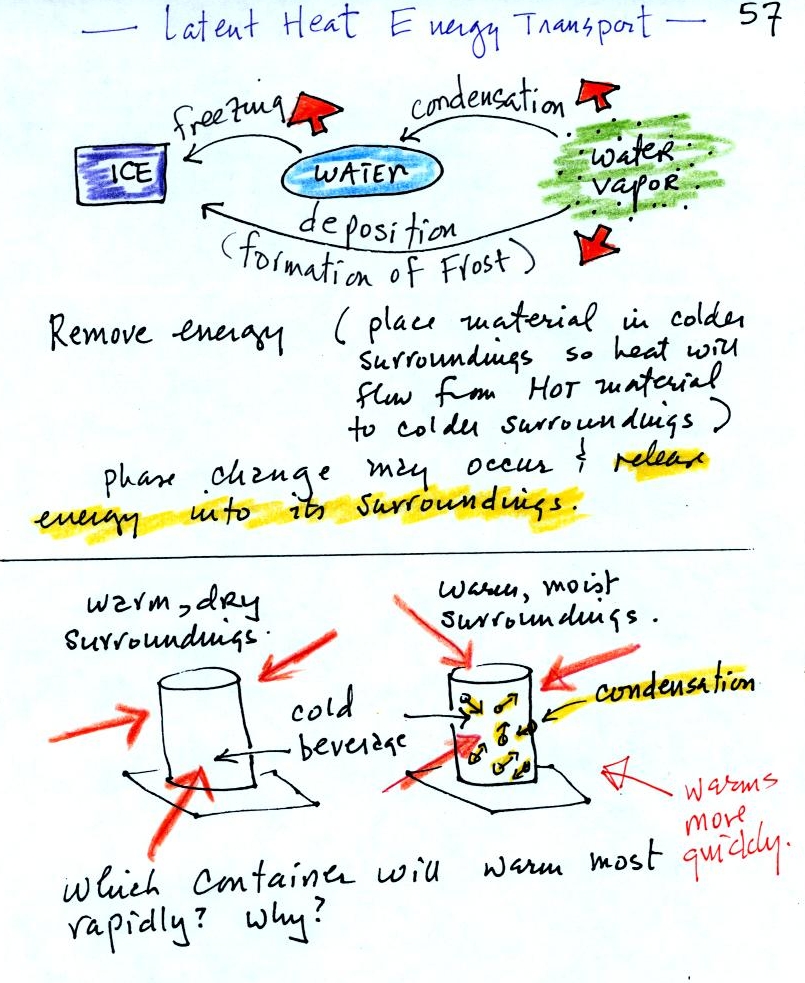
Latent
heat energy transport was the next topic of the day.
Energy
transport in the form of latent heat is the second most important
energy transport process (second only to electromagnetic
radiation).
If you had an object that you wanted to cool off quickly you could blow
on it. Or you could stick it into some water, that would cool it
off pretty quickly. You'd here a brief sizzling sound, the sound
of boiling water. A lot of energy would be taken quickly from the
hot object and used to boil a small amount of water.
Latent heat energy transport is sometimes a little hard to visualize
or understand because the energy is "hidden" in water vapor or water.
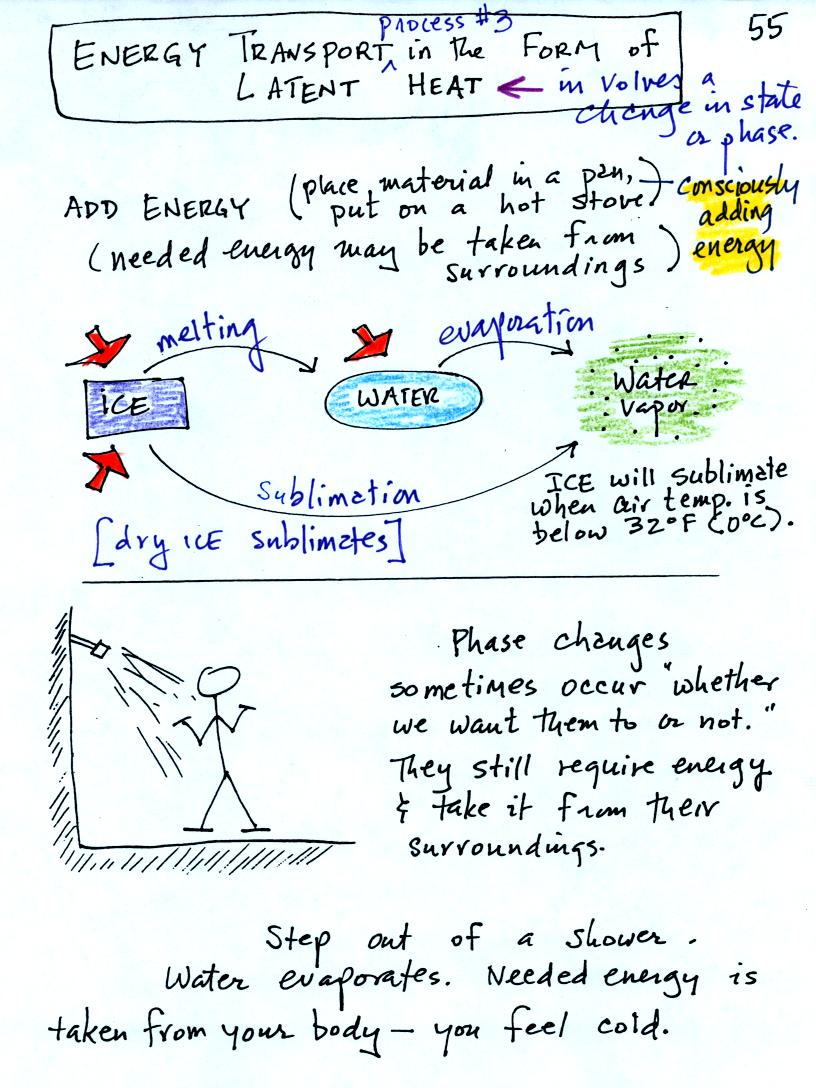
Latent heat energy transport is
associated with changes of
phase (solid to liquid, water to water vapor, that sort of thing) A
solid to liquid phase change is melting, liquid to gas is
evaporation, and sublimation is a solid to gas phase change (dry ice
sublimates when placed in a warm room, it turns directly from solid
carbon dioxide to gaseous carbon dioxide).
In
each case energy must be added to the material changing phase.
You can consciously add or supply the energy (such as when you put
water in a
pan and put the pan on a hot stove) or the needed energy will be
taken from the surroundings (from your body when you step out of a
shower in the morning).
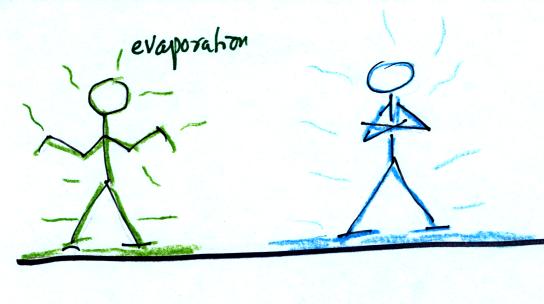
When
your body starts to lose energy, it feels cold.
A 240 pound man or woman running at 20 MPH has just
enough
kinetic energy (if you could capture it) to
be able to melt an ordinary ice cube. It would take 8 people
running at 20 MPH to
evaporate the resulting water.
You can consciously remove energy from water vapor to make
it
condense
or from water to cause it to free (you could put water in a
freezer; energy would flow from the relatively warm water to the
colder surroundings). Or if one of these phase
changes occurs energy will be released into the surroundings (causing
the surroundings to warm). Note the orange energy arrows have
turned around and are pointing from the material toward the
surroundings.
A can of cold drink will warm more quickly in warm moist surroundings
than in warm dry surroundings. Heat will flow from the warm air
into the cold cans in both cases. Condensation of water vapor is
an additional source of energy and will warm that can more
rapidly. The condensation may actually be the dominant process.
You feel cold when you step out of a shower and water on
your body
evaporates. The opposite situation, stepping outdoors on a humid
day
and actually having water vapor condense onto your body (it can happen
to your sunglasses but not to you, your body is too warm). If it
did
happen it would warm you up.
This figure shows how energy can be transported from one
location to another in the form of latent heat. The story starts
at left in the
tropics where there is often an abundance or surplus of sunlight
energy. Some of the incoming
sunlight
evaporates ocean water. The resulting water vapor moves somewhere
else and carries hidden latent heat energy with it. This hidden energy
reappears when something (air running into a mountain and rising,
expanding, and cooling) causes the water vapor to condense. The
condensation releases energy into the surrounding atmosphere.
Energy arriving in sunlight in the tropics has effectively been
transported to the atmosphere in Tucson.
The following figure
wasn't shown
in class.
The formation of a cloud means that
latent heat is being released into the air. Two energy transport
processes are at work in this picture: convection and latent heat
(conduction is also present, that is how is energy is transported from
the hot ground into the thin layer of air in contact with the
ground. In this case energy is being transported vertically in
the form of latent heat.
We'll
spend the next two or three class periods on electromagnetic
(EM) radiation. It is the most important energy transport process
because it can travel through empty space. The notes that follow are a little more
detailed that was done in class.
To really understand EM radiation you need to understand
electric
fields. To understand electric fields we need to quickly review a
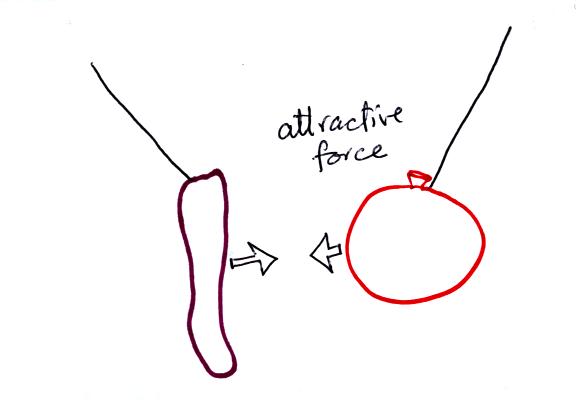
basic rule concerning static electricity.
Static electricity was demonstrated (one
of the poorer demonstrations of the semester) using a sweater
(a gift from my Aunt Ethel and Uncle Nelson made of acrylic
fiber and wool) and two balloons.
Each balloon was rubbed with the sweater. The
balloons (and the sweater) became electrically charged (the balloons
had one polarity of charge, the sweater had the other).
We didn't know what charge
the balloons carried just that they both had the same charge.
If you bring the balloons close to each other they are
pushed apart by
a repulsive electrical force.
The sweater and the balloon carry opposite
charges. IF
they are
brought together they experience an attractive electrical force.
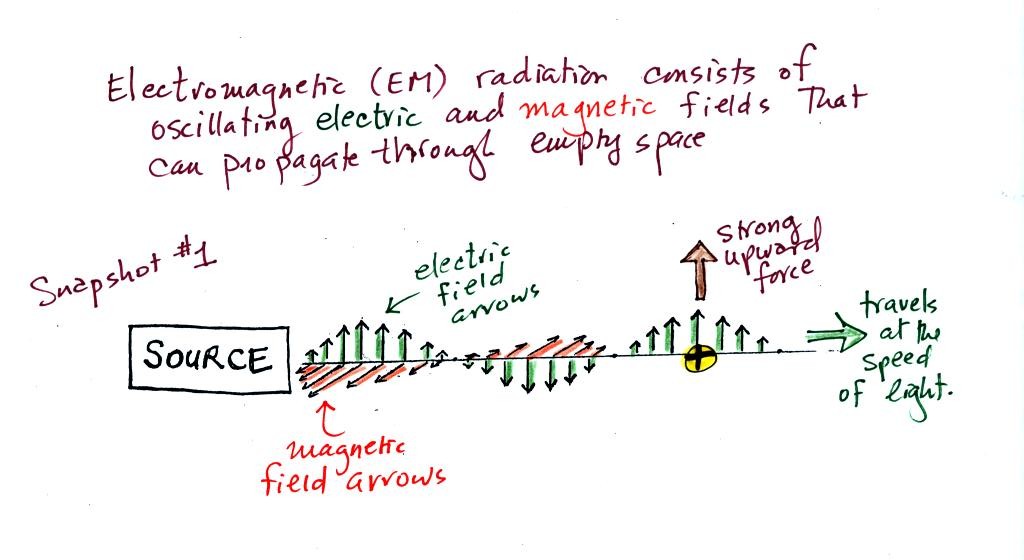
Our next step in understanding EM is to learn something
about electric
field arrows. Imagine placing a + charge at the three
positions shown in the figure
below.
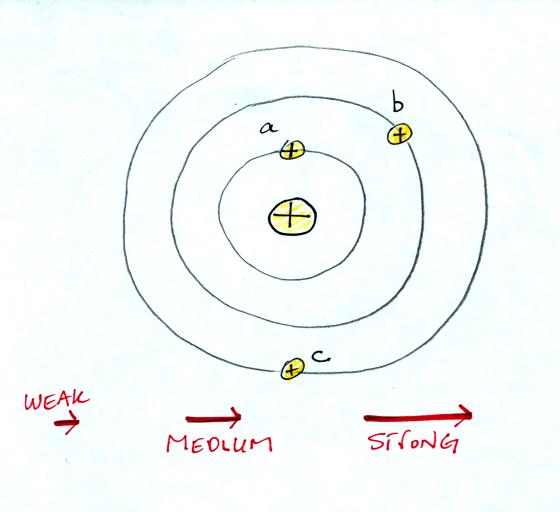
Then choose one of the three arrows
at the bottom of the picture to show both the direction and the force
that would be exerted on each charge.
Here's the answer. The closer
the charge is to the center, the greater the strength of the outward
force. With just a little thought you can see that if you were to
place + charges at other
positions you would quickly end up with a
figure that looks like the pattern at the bottom of p. 59 in the
photocopied ClassNotes.
The electric field arrows in this
picture show the direction and give an idea of the strength that would
be exerted on a positive placed at any position in the figure.
You'll find the following on p. 60 in the photocopied ClassNotes.
We imagine turning on a source of EM radiation and then
a
short time
later we take a snapshot. The EM radiation is a wavy pattern of
electric and magnetic field arrows. We'll ignore the magnetic
field lines. The E field lines sometimes point up, sometimes
down. The pattern of electric field arrows repeats itself.
Note the + charge near the right
side of the picture. At the time
this
picture was taken the EM radiation exerts a fairly strong upward force
on the + charge.
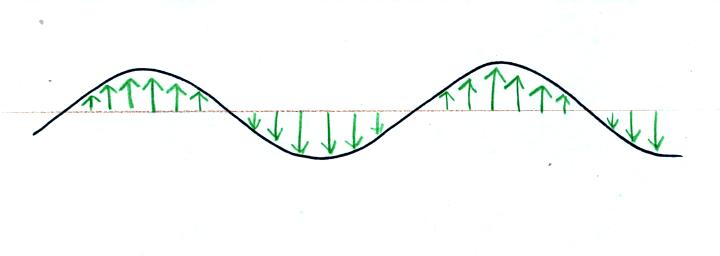
Textbooks often represent EM radiation with a wavy line like shown
above. But what does that represent?
The wavy line just connects the
tips of a bunch of electric
field
arrows.
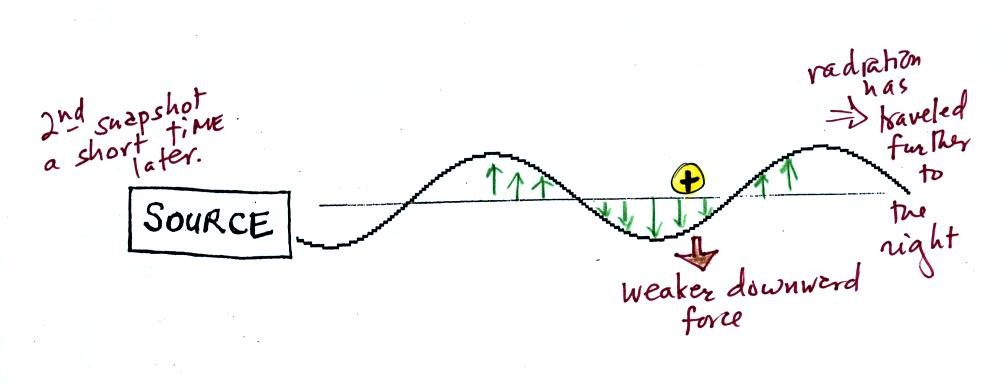
This picture was taken a short time after the first snapshot when
the radiation
had
traveled a little further to the right. The EM radiation now
exerts a somewhat weaker downward force on the + charge.
The + charge is now being
pushed upward again. A
movie
of
the +
charge, rather than just a series of snapshots, would show the
charge
bobbing up and down much like a swimmer in the
ocean would do as waves passed by.
The wavy pattern used to
depict EM radiation can be described spatially in terms of its
wavelength,
the distance between identical points on the pattern. By
spatially we mean you look at different parts of the radiation at one
particular instant frozen in time.
Or you can
describe the radiation temporally
using the frequency of oscillation
(number of up and down cycles completed by an oscillating charge per
second). By temporally we mean you at one particular point for a
certain period of time.
Here are the answers (in red) to the series of questions shown in
class.

1. What two phase changes are occurring in the
picture?
(you might not be able to see them, also the cloud you see is not
carbon dioxide gas) The dry ice is first
of all sublimating (turning from solid to gas). The sublimation
is invisible. The cloud that you do see is composed of water droplets
or ice crystals. Water vapor coming into contact with the cold
dry ice condenses to form water droplets (or perhaps ice crystals).
Is energy being transported from the surroundings INTO the dry
ice or
AWAY from the dry ice and into the surrounding air? Energy flows from
the warmer surrounding air into the much colder dry ice. That is
what cools the air enough for the cloud to form and become
visible. The condensation that occurs as the cloud forms also
releases hidden latent heat energy which goes into the dry ice.
2. The person is trying to cool the hot steaming bowl of
soup
by blowing
on it.
What two energy transport processes are at work
here?
(conduction is not one of the answers I'm looking for)
Blowing on the bowl
of soup is forced convection. The hot soup is also evaporating
(the soup is steaming hot). The energy needed for water in the
soup to evaporate is taken from the soup and cools the soup (just like
the water that evaporates off your wet body when you step out of a
shower takes energy from your body and makes you feel cold).
3. A person is standing outside on a cold windy day in A, has
fallen into cold water in B, and is perspiring heavily in C.
Match each energy transport process below with the most appropriate
situation in the drawing.
Conduction___B___
Convection ___A___
Latent
heat ___C____
4. Would the formation of a
cloud WARM
or COOL the
surrounding air? Water vapor
condenses to form the cloud. Latent heat energy is released into
the surrounding air when water vapor condenses.
Does the formation of frost WARM
or COOL the
air? This
is a similar situation. Latent heat energy is released into the
surroundings as water vapor changes directly to ice (deposition).