Monday Oct. 12, 2009
click here to download today's notes in
a more printer friendly format
Isn't Oct. 12 Columbus Day? Shouldn't we have had the day off?
Some what I guess might be called Rockabilly music before class today:
"Hot Rod Lincoln" (part
1 and part 2)
and "I Gotta Get Drunk" from the Twangbangers.
The Experiment #2 reports were collected in class today. It takes
about a week to get those graded. I'm guessing you should expect
to get them back next Wednesday Oct. 21. You will then have a
chance to revise your reports if you want to.
I am hoping to distribute the Expt. #3 materials in class on Friday.
Class
began with a demonstration, one that might save students (that live off
campus and
pay electric bills) some money.
Last Friday we learned that ordinary tungsten bulbs (incandescent
bulbs) produce a lot of
wasted energy. They emit a lot of infrared light that is
wasted because it doesn't light up a room (it will heat up a room but
there are better ways of doing that). The light that they do
produce is a warm white color (tungsten bulbs emit lots of orange, red,
and yellow light and not as much blues, greens and violets). Energy
efficient
compact
fluorescent lamps (CFLs) are
being touted as an ecological alternative to tungsten bulbs because
they use substantially less electricity, don't emit a lot of
wasted infrared light, and also last
longer. CFLs come with
different color temperature ratings.
The bulb with the hottest
temperature rating (5500 K ) in the figure
above is meant to mimic or simulate sunlight. The temperature of
the sun is 6000 K and lambda max is 0.5 micrometers. The spectrum
of the 5500 K bulb is similar.
The tungsten bulb (3000 K) and the CFLs with temperature ratings
of
3500 K and 2700 K produce a warmer white.
Three CFLs with the temperature ratings above were set up in class
so
that you could see the difference between warm and cool white
light. Personally I find the 2700 K bulb "too warm," it makes a
room
seem gloomy at night. The 5500 K bulb is "too cool" and creates
a stark sterile atmosphere like you might see in the hallways in a
hospital. I prefer the 3500 K bulb in the
middle.
This figure below is from an article
on compact fluorescent lamps in Wikipedia for those of you that weren't
in class and didn't see the bulb display.. You can
see a clear difference between the cool white bulb on the left
in the figure below and the warm white light produced by a tungsten
bulb (2nd from the left) and 2 CFCs with low temperature ratings (3rd
and 4th from the left).
There is one downside to these energy efficient CFLs. The bulbs
shouldn't just be discarded in your ordinary household trash because
they contain mercury. They should be disposed of properly.
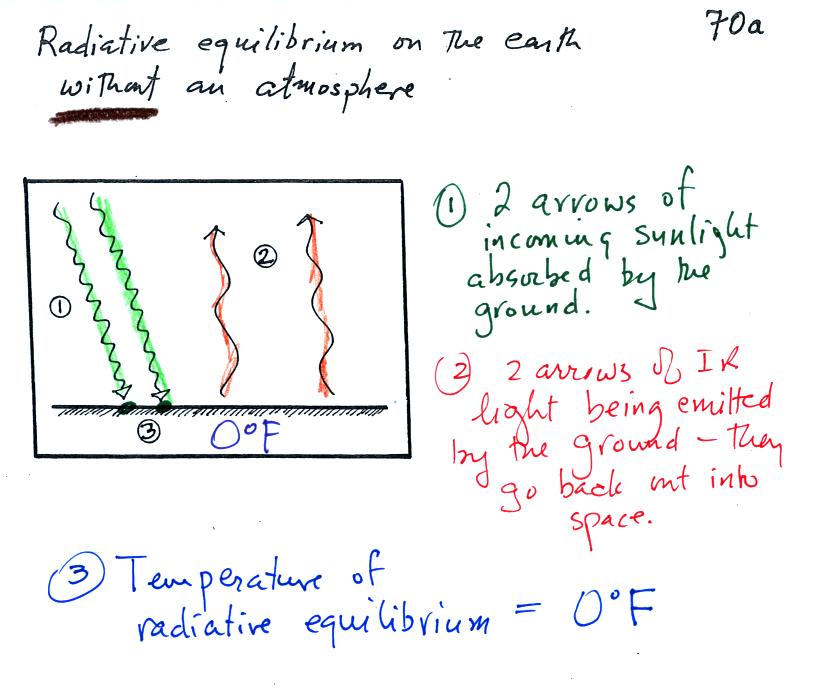
We looked at radiative equilibrium on the earth without an
atmosphere in class last Friday. Here is what that looked liked.
Look closely at the picture. 5 arrows (5 units) of sunlight
energy arrive at the earth. 4 arrows are absorbed, one is
reflected. The earth is in energy balance because it is emitting
the same amount of energy (4 arrows). The earth doesn't have to emit
the same kind, just the same amount to be in energy balance.
Today we will look at energy balance on the earth with an
atmosphere. This is where the atmospheric greenhouse effect will
show up.
Before we
start to look at radiant energy balance on the earth with an atmosphere
we
need to learn about filters. The atmosphere will filter sunlight
as it
passes through the atmosphere toward the ground. The atmosphere
will
also filter IR radiation emitted by the earth as it trys to travel into
space.
We will first look at the effects simple blue, green, and red glass
filters have on visible light. This is just to become familiar
with filter absorption graphs.
If you try to shine white light (a
mixture of all the colors) through a
blue filter, only the blue light passes through. The filter
absorption curve shows 100% absorption at all but a narrow range of
wavelengths that correspond to blue light. Similarly the green
and red filters only let through green and red light.
The following figure is a simplified, easier to
remember,
representation of the
filtering effect of
the atmosphere on UV, VIS, and IR light (found on p. 69 in the
photocopied notes). The figure was borrowed from a previous
semester because it was drawn more neatly.
You can use your own eyes to tell
you what the filtering
effect of the
atmosphere is on visible light. Air is clear, it is
transparent. The atmosphere transmits visible light.
In our simplified representation oxygen and ozone make the
atmosphere pretty nearly completely opaque to UV light . Don't
let the word opague bother you - we assume that the
atmosphere absorbs all incoming UV light, none of it makes it to the
ground. This is of course not entirely realistic.
Greenhouse gases make the
atmosphere a
selective absorber of IR light - the air absorbs certain IR wavelengths
and
transmits others. It is the atmosphere's ability to absorb (and
also emit) certain wavelengths of infrared light that produces the
greenhouse effect and warms the surface of the earth.
Note "The atmospheric window"
centered at 10 micrometers. Light emitted by the earth at this
wavelength will pass through the atmosphere. Another transparent
region, another window, is found in the visible part of the spectrum.
You'll find a more realistic picture of the atmospheric absorption
curve on p. 70 in the photocopied Classnotes, but the simplified
version above will work fine for us.
Here's the
outer space view of radiative equilibrium on the earth without an
atmosphere. The important thing to note is that the earth is
absorbing and emitting the same amount of energy (4 arrows absorbed
balanced by 4 arrows emitted).
We will be moving from an outer
space vantage point of
radiative equilibrium (figure above) to the earth's
surface (figure below).
Don't let the fact that there are
4 arrows are
being absorbed and
emitted in the top figure and
2 arrows absorbed and emitted in the bottom figure
bother you. The important thing is that there are equal
numbers of arrows coming in and going out. That is what indicates
energy balance. Balance occurs then the earth has warmed to 0 F.
The next
step is to add the atmosphere.
We will study a simplified version
of radiative equilibrium just so you
can identify and understand the various parts of the picture.
Keep an eye out for the greenhouse effect. Here's the figure that
we ended up with in class
It would be hard to sort through all of this if you weren't in
class
(and maybe even if you were) to see how it developed. So below we
will go through it again step by step (which you are free to skip over
if you wish). Caution: some of the colors below are different
from used in class.
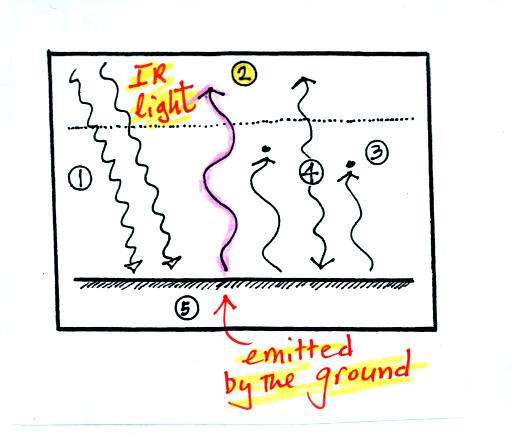
1. The
figure shows two
rays of incoming sunlight that
pass through the atmosphere, reach the ground, and are absorbed.
100% of the incoming sunlight is transmitted by the atmosphere.
This wouldn't be too bad of an assumption if sunlight were just visible
light. But it is not it is about half IR light and some of that
is going to be absorbed.
The ground is emitting 3 rays of IR radiation.
2. One of these is emitted by
the ground at a wavelength
that is
NOT absorbed by greenhouse gases in the atmosphere. This
radiation passes through the atmosphere and goes out into space.
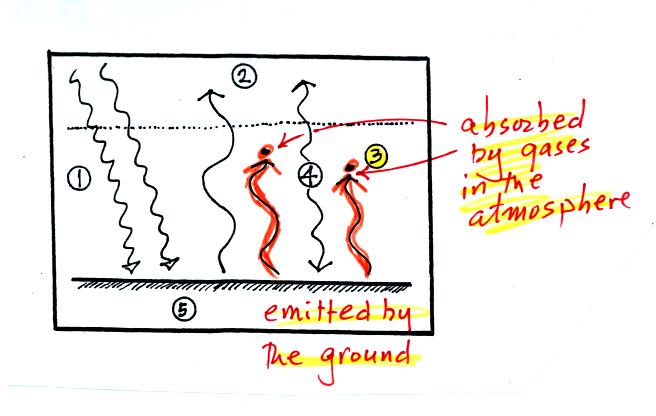
3. The other 2 units of IR radiation emitted by
the
ground are
absorbed by
greenhouse gases is the atmosphere.
4. The atmosphere is absorbing
2 units of radiation.
In order to be in radiative equilibrium,the atmosphere must also emit 2
units of radiation. 1
unit of IR radiation is sent upward into space, 1 unit is sent downward
to the ground where it is absorbed.
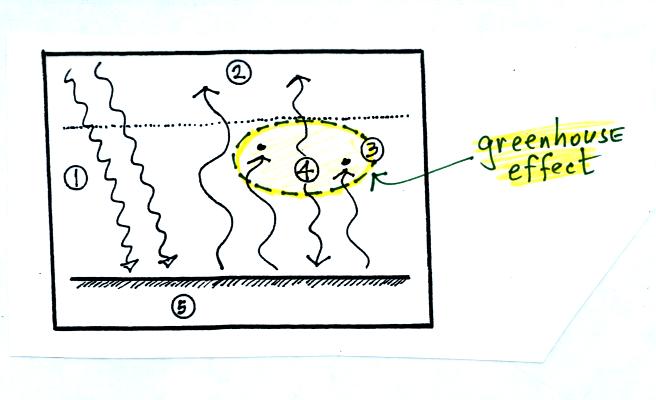
The greenhouse effect is found in this absorption and
emission
of IR radiation by the atmosphere. Here's how you might put it
into words:
Before we go any further we will check
to be sure that
every part
of this picture is in energy balance.
The ground is absorbing 3 units of energy (2 green
arrows and one purple arrow above) and emitting
3
units of energy (one pink and two red arrows)
The atmosphere is absorbing 2 units of energy and
emitting 2
units of
energy
2 units of energy arrive at the earth from outer
space, 2 units
of
energy leave the earth and head back out into space.
The
greenhouse effect makes the earth's surface warmer than it would be
otherwise (global annual average of 60 F instead of 0 F).
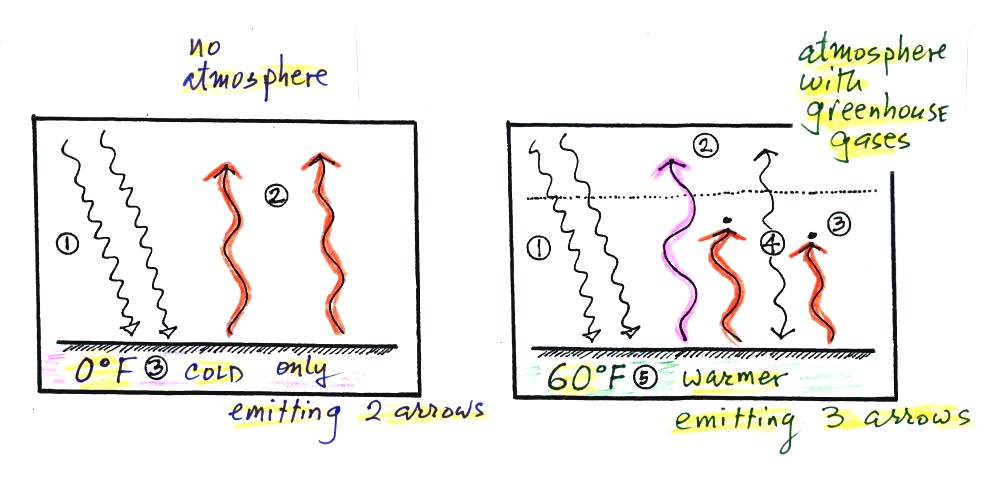
Energy balance with (right) and without (left) the
greenhouse
effect. At left the ground is emitting 2 units of energy, at
right the ground is emitting 3 units. Remember that the amount of
energy emitted by something depends on temperature. The ground
must be warmer to be able to emit 3 arrows of energy rather than 2
arrows.
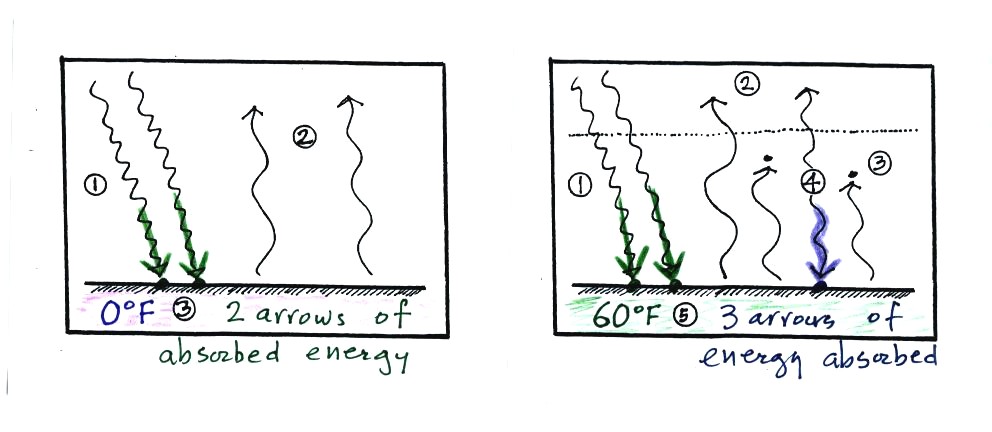
Here's another explanation (that wasn't mentioned in class).
At left the ground
is getting 2 units of energy. At right it is getting three, the
extra one is coming from the atmosphere. Doesn't it seem
reasonable
that ground that absorbs 3 units of energy will be warmer than ground
that is only absorbing 2?
We did just a little bit more in class. The following material
won't be on this week's quiz.
In
our simplified explanation of the greenhouse effect we assumed that
100% of the sunlight arriving at the earth passed through the
atmosphere and got absorbed at the ground. Next we will look at how
realistic that assumption is.
The bottom figure
above shows that in reality only about 50% of the incoming sunlight
gets absorbed at the ground.
About 20% of the incoming sunlight is absorbed by gases in the
atmosphere. Sunlight is a
mixture of UV, VIS, and IR light.
Ozone and oxygen will absorb a lot of the UV (though there isn't much
UV in sunlight) and greenhouse gases will absorb some of the IR
radiation in sunlight (Roughly half of sunlight is IR light).
The remaining 30% of the incoming sunlight is reflected or
scattered back into
space
(by the ground, clouds, even air molecules).
Next we
will look at our simplified version of radiative equilibrium and a more
realistic picture of the earth's energy budget. Here's a figure
from a previous semester.
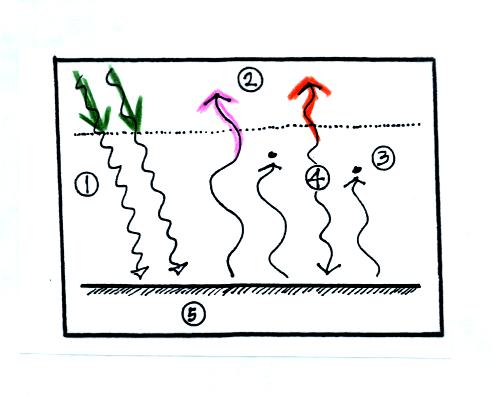
In the top figure you should recognize the incoming sunlight
(green),
IR emitted by the ground that passes through the atmosphere (pink), IR
radiation emitted by the ground that is absorbed by greenhouse gases in
the atmosphere (orange) and IR radiation emitted by the atmosphere
(dark blue). Using the colors you can find each of these parts of
the energy budget in the bottom figure. Notice that conduction,
convection, and latent heat energy transport are needed to bring the
overall energy budget into balance. The amount of energy transported by
conduction, convection, and latent heat is small compared to what is
transported in the form of EM radiation.
The lower part of the figure is
pretty complicated. It
would be
difficult to start with this figure and find the greenhouse effect in
it. That's why we used a simplied version. Once you
understand the upper figure, you should be
able to find and understand the corresponding parts in the lower figure.
Two or three things to just note in the bottom figure. First
the ground receives more energy from the atmosphere (96 units) than it
gets from the sun (51 units). The ground emits more energy (117
units) than it gets from the sun (51 units). It is able to
achieve energy balance because it gets lots of energy from the
atmosphere. The atmosphere emits 64 units upward and 96 units
downward. This might be explained by the lower atmosphere being
warmer than higher up in the atmosphere. Or it might just that
there is more air in the bottom of the atmosphere than near the top of
the atmosphere.
We have a few relatively minor points to finish up on this topic on
Friday and then it will be on to something new again.