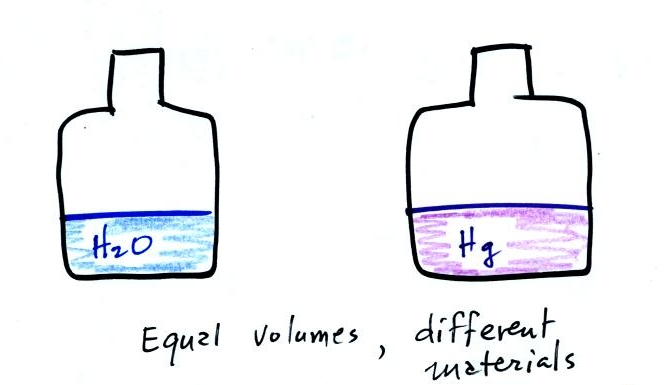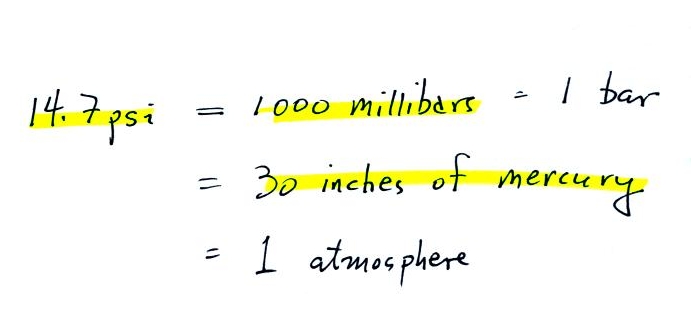Friday Sep. 11, 2009
click here to download today's notes in
a more printer friendly format
A couple of songs ( "Calloway Boogie" and "Go Daddy-O" )
from Big Bad
Voodoo Daddy before class today. A local group, Calexico, will be
featured next week, all week.
The Practice Quiz has been graded and was returned in class
today. I would suggest you read through the answers and comments. The
average 66% is a little bit higher than average for a Practice
Quiz. If you did study for this quiz and didn't do as well as you
expected to come by my office sometime and we'll try to figure out what
happened.
Be sure to keep any papers that are returned to you until the semester
is over and you have received a grade for this class. This is
just in case you think an error has been made in computing your
grade. Grades for this class aren't posted online (mostly because
I'm not sure how to do that and keep the information
confidential). You will get a grade summary or two during the
semester and are welcome to come by my office to check on your grade.
The first of this semester's 1S1P
Assignments
is now online. The Bonus Report is due next Thursday, Sep.
17. If you decide to do a report on Topic #1 or #2 (or both),
those reports are due on Tue., Sep 29. Be sure to keep a copy of
any reports that you turn in just in case something is lost.
Please go back and read the last portion of the Wednesday
Sep. 9 class notes. There was a little bit of information
added after class.
So far
this semester we have learned about the composition of the atmosphere
and about some of the main air pollutants. Today we will start
looking
at how atmospheric characteristics such
as
air temperature, air pressure, and air density change with
altitude. In the case of air pressure we first need to understand
what pressure is and why it changes as you move vertically through the
atmosphere.
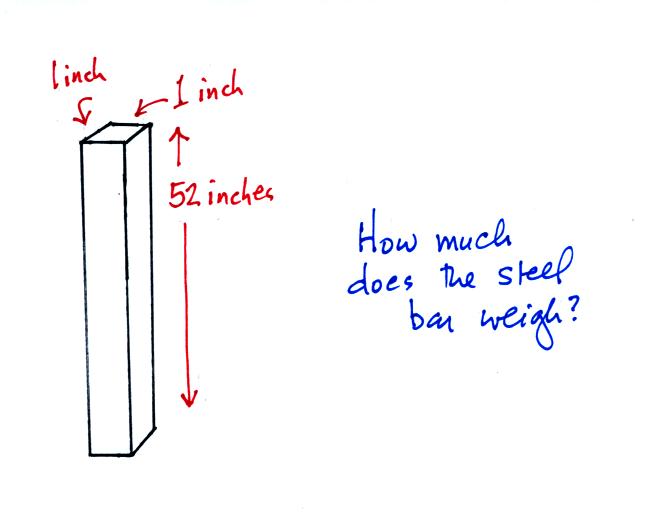
An iron bar was passed around at the
beginning of class. You were supposed to guess how much it
weighed.
We came back to this later in the period.
A pair of bottles, one containing water and the other
an equal volume of mercury, were also passed around in class. Feel the
difference in the weights of the two bottles. Mercury is much
denser than water.
Just to make my life easier and to get these notes online before 5
pm (so I don't have to come into my office over the weekend), I've
basically copied the notes from the Sect. 3 class. Some of the
figures below may be slightly different from what was done in class
today.
Before we can learn about
atmospheric pressure, we
need to review
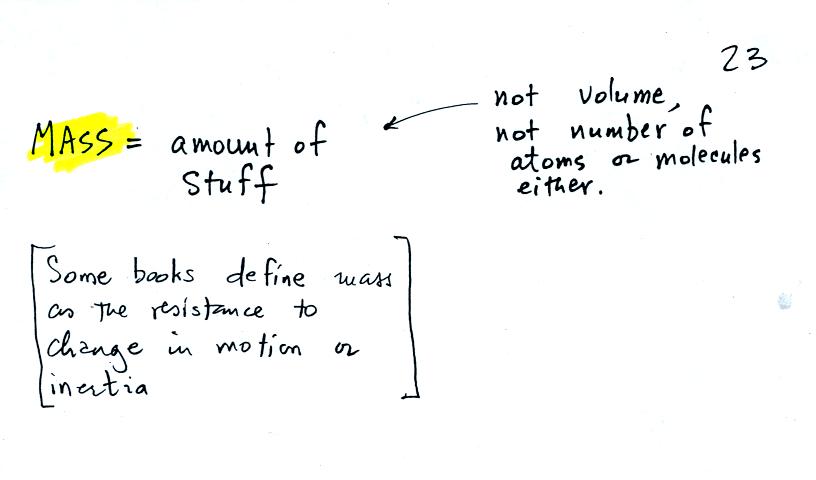
the terms mass and weight. In some textbooks you'll find mass
defined as "amount of stuff" or "amount of a particular
material." Other books will define mass as
inertia or as resistance to change in motion (this comes from Newton's
2nd law of motion, we'll cover that later in the semester). The
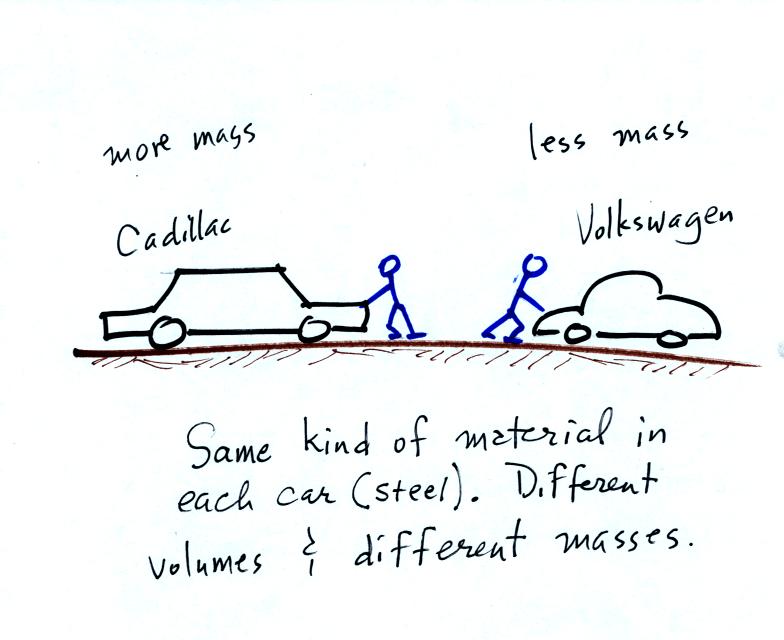
next picture
illustrates both these definitions. A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down once it is
moving).
Differences in volumes account for the differences in mass in the
example above. It is possible to have two objects with the
same
volume but very
different masses. The bottles of water and mercury that were
passed around class were an example (thanks for being careful
with the mercury).
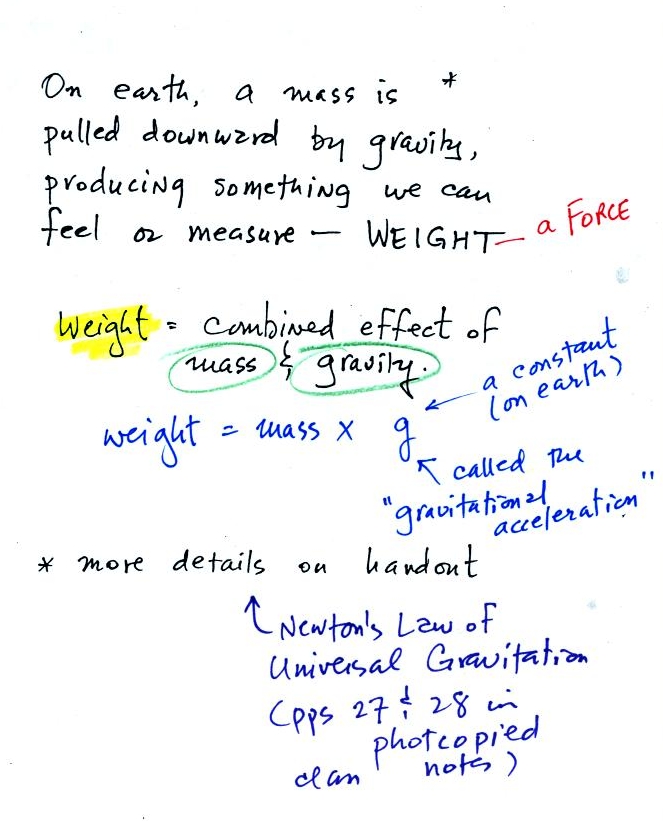
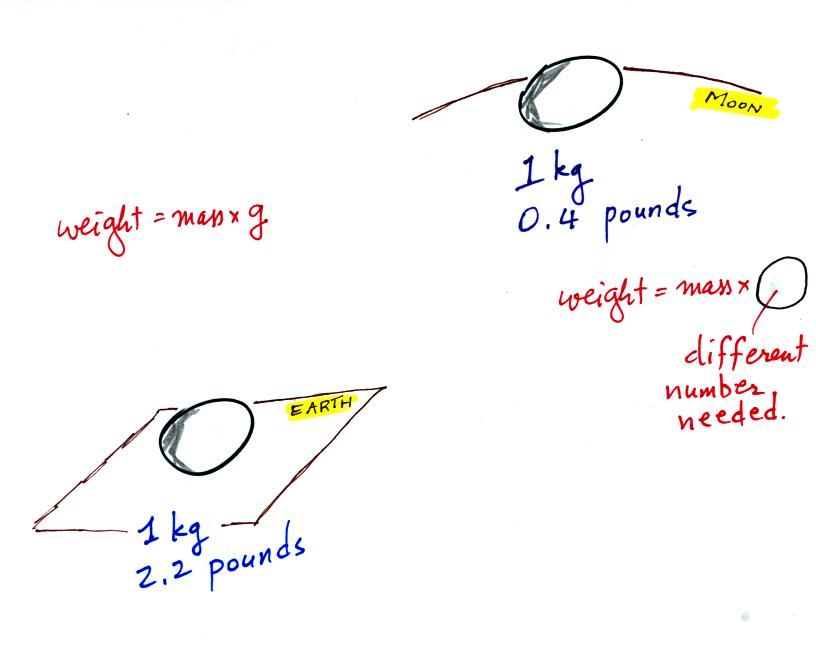
Weight
is a force and depends on
both the mass of an object and the
strength of gravity. We tend to use
weight and mass
interchangeably
because we spend all our
lives on earth where gravity never changes.
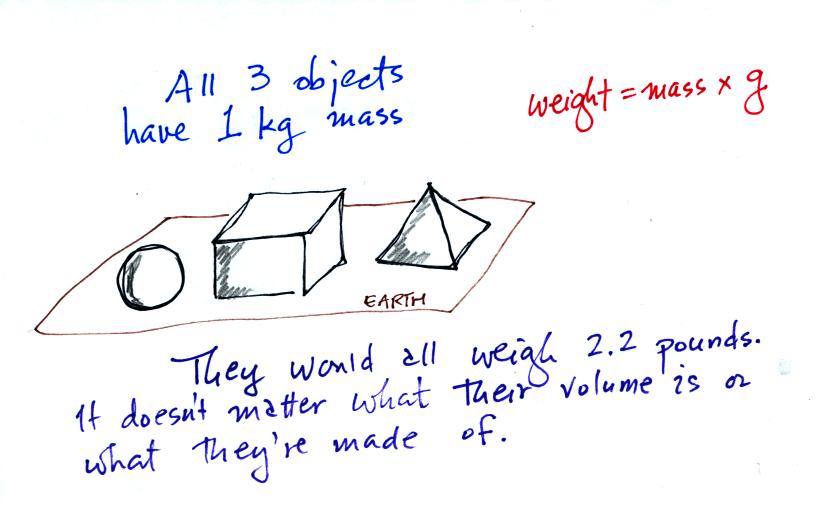
On the earth where the pull of gravity never changes, any three objects
that all have the same mass
(even if they had different volumes and were made of different
materials) would always have the same weight. Conversely:
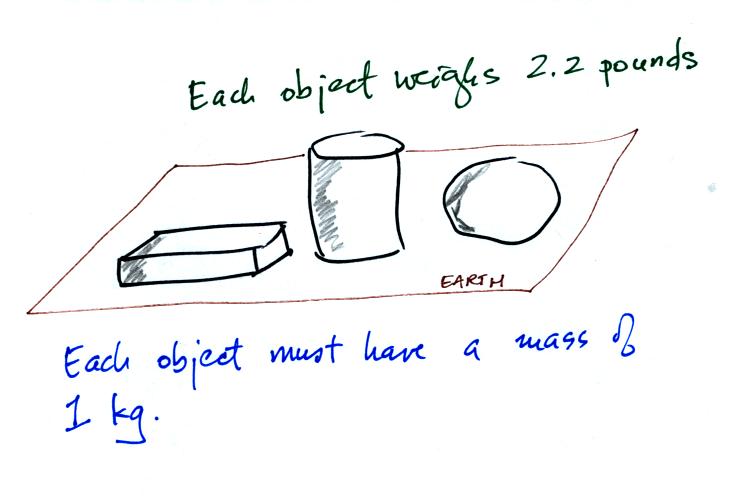
When gravity is always the
same, three
objects with the
same weight
would also have the same mass.
The difference between mass and weight is clearer
(perhaps) if you
compare the situation on the earth and on the moon.
If you
carry an object
from the
earth to the moon, the mass
remains the
same (it's the same object, the same amount of stuff) but the weight
changes because gravity on the moon is weaker than on the earth.
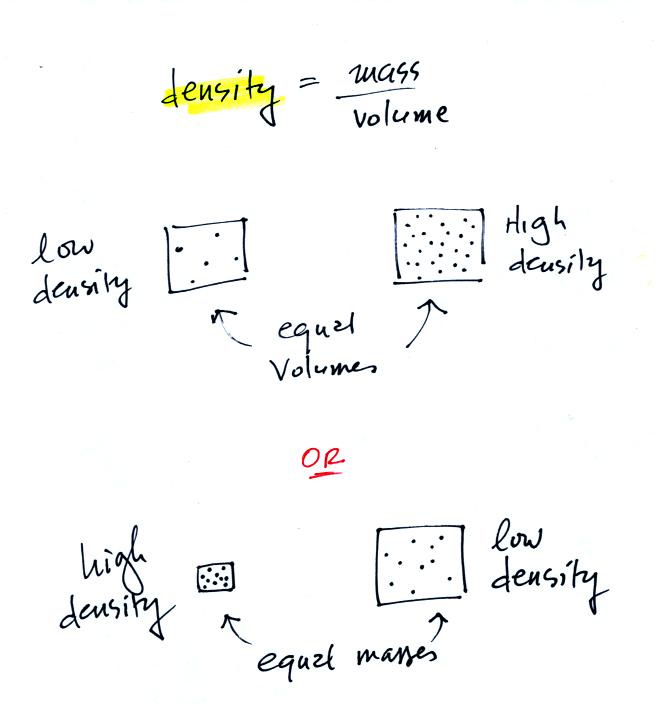
In
the first example there is more mass (more dots) in the right box than
in the left box. Since the two volumes are equal the box at right
has higher density. Equal masses are squeezed into different
volumes in the bottom example. The box with smaller volume has
higher density.
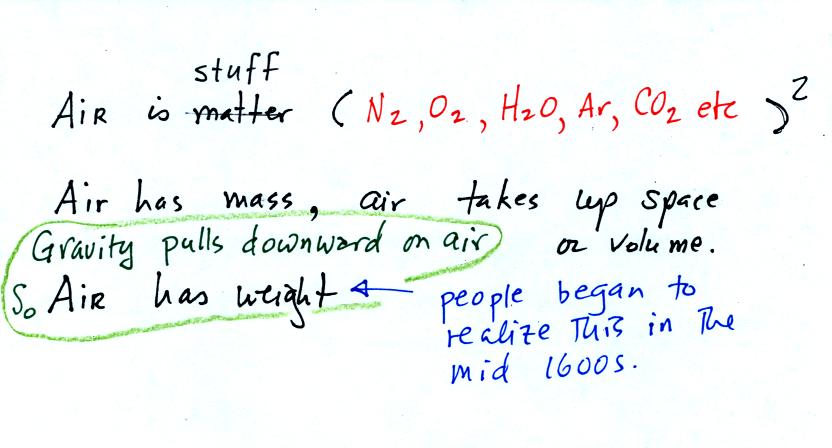
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment to prove that air has weight. That experiment wasn't mentioned in
class.
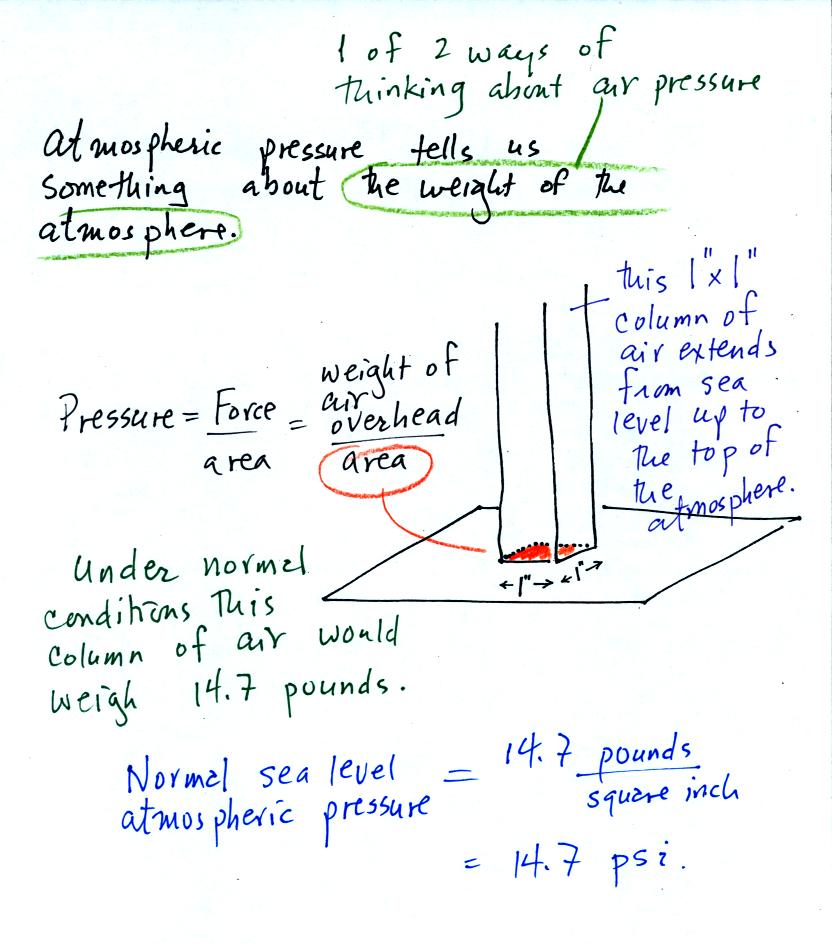
Pressure is defined as force divided by area. Air
pressure is the
weight
of the atmosphere overhead divided by the area the air is resting
on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead. This is one way, a sort of large scale representation,
of understanding air pressure.
Under normal conditions a 1 inch by 1 inch column of air
stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your car or bike tires with air).
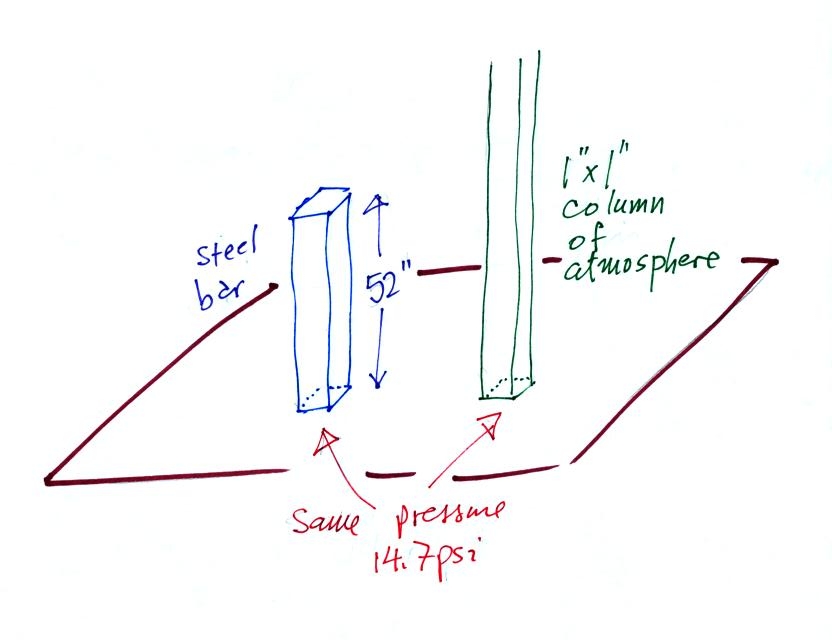
Now here's where the steel bar
comes in. The steel bar also weighs exactly 14.7 pounds (many
people think it is heavier than that). Steel is a lot denser
than air, so a steel bar only needs to be
52 inches tall to have the same weight as an air column that is 100
miles or more tall.
Here are some of the other commonly used pressure
units.
Typical sea level
pressure is 14.7 psi or about 1000 millibars
(the
units used by meterologists and the units that we will use in this
class most of the time) or about 30 inches of mercury (refers to
the reading on a mercury barometer). If you ever find
yourself in France needing to fill your
automobile tires with air (I lived in France for a while and owned
a Peugeot
404)
remember that the air compressor scale is
probably calibrated in bars. 2 bars of pressure would be
equivalent to 30 psi.
The word "bar" basically means
pressure and is used in a lot of meteorological terms.
Pressure
at sea level is determined by the weight of the air overhead.
What about pressure at some level above sea level?
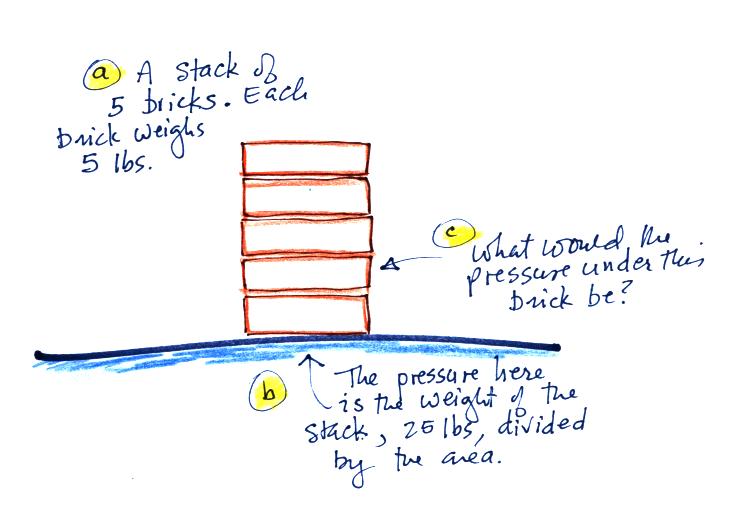
We can use a stack of bricks to try to
answer this
question.
Each brick
weighs 5 pounds. At the bottom of the 5 brick tall pile you would
measure a weight of 25 pounds. If you moved up a brick you would
measure a weight of 20 pounds, the weight of the four bricks still
above. To get the pressure you would need to divide by the
area. It should be clear that weight and pressure will decrease
as you move up the pile.
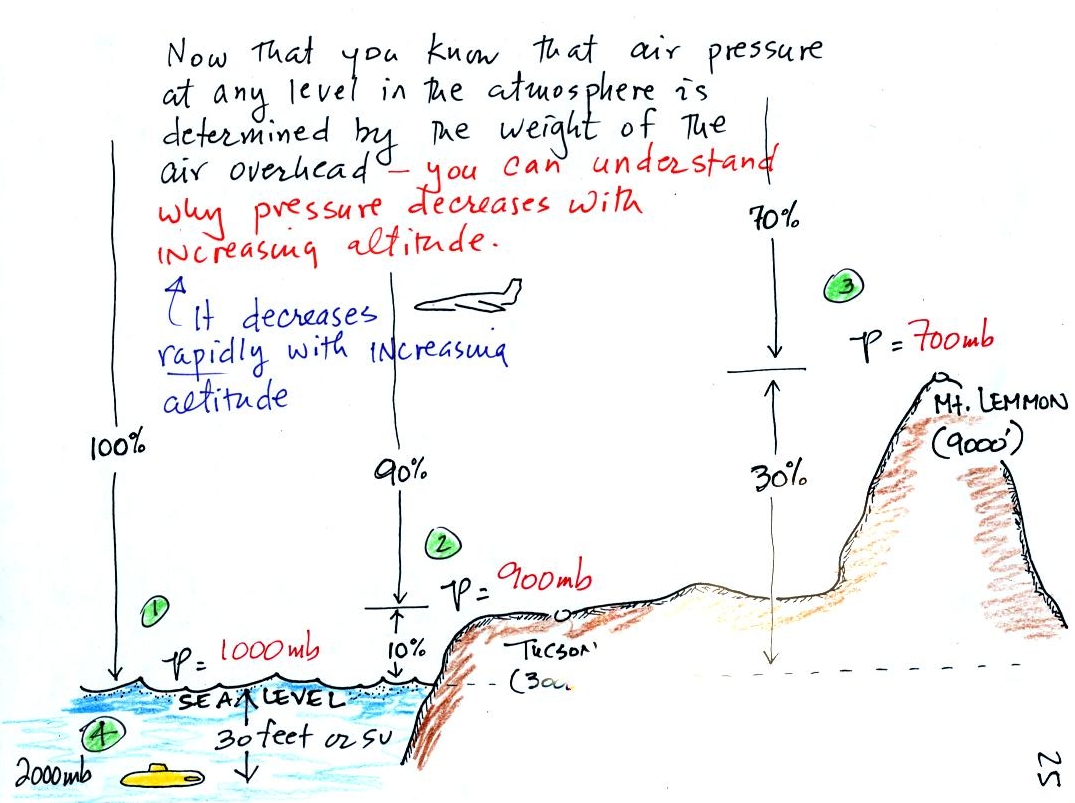
In the atmosphere, pressure at any level is determined by
the weight of the air still overhead. Pressure decreases with
increasing altitude because there is less and less air remaining
overhead. The numbered points on the figure below were added
after class.
At sea
level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all (100%) of the air in the atmosphere.
Some parts of Tucson, at Point 2, are 3000
feet above sea level (most
of the valley is a little lower than that around 2500 feet). At
3000 ft. about 10%
of the
air is
below, 90% is still overhead. It is the weight of the 90% that is
still above that determines the atmospheric pressure in Tucson.
If 100% of the atmosphere produces a pressure of 1000 mb, then 90% will
produce a pressure of 900 mb.
Pressure is typically about 700 mb at the
summit of Mt. Lemmon (9000
ft. altitude at Point 3) and
70% of the atmosphere is overhead..
Pressure decreases rapidly with increasing
altitude. We will find that pressure changes more slowly if you
move horizontally. It is small horizontal changes that cause the
wind to blow however.
Point 4 shows
a submarine at a depth of
about 30 ft. The pressure
there is determined by the weight of the air and the weight of the
water overhead. Water is much denser and much heavier than
air. At 33 ft., the pressure is already twice what it would be at
the surface of the ocean (2000 mb instead of 1000 mb).
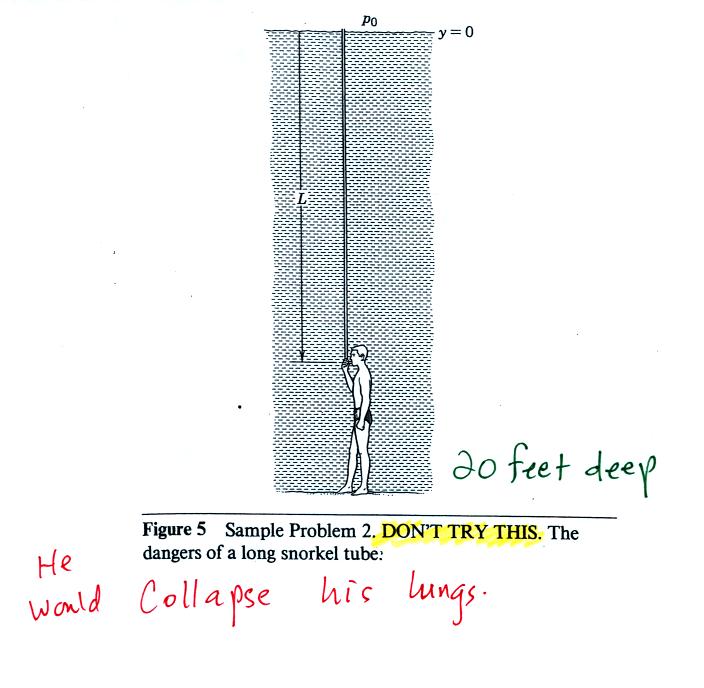
The person in the picture below (from a
Physics textbook) is 20 feet
underwater. At that depth there is a pretty large pressure
pushing against his body
from the surrounding water. The top of the snorkel is exposed to
the much lower air pressure at the top of the pool. If the
swimmer puts his mouth on the snorkel the pressure at the bottom of the
pull would collapse his lungs.