Lecture notes on the ozone hole from a previous semester of NATS
101 (click here to download
in a more printer friendly format)
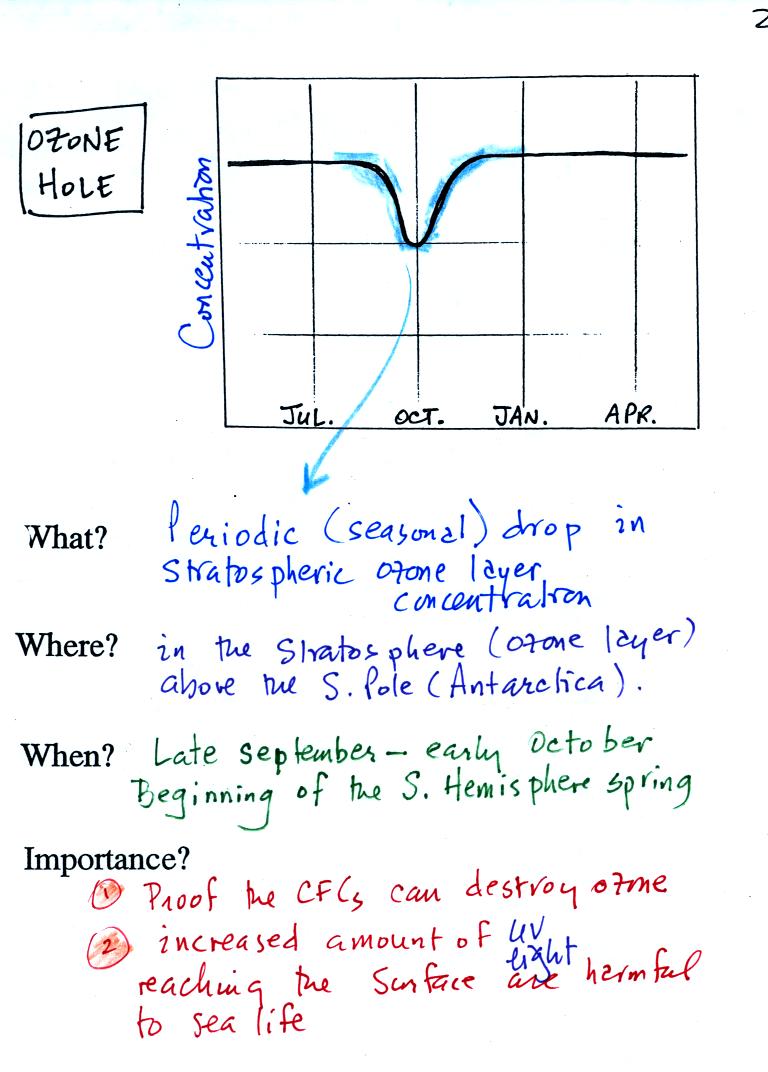
The ozone hole that forms above the
S. Pole every year in late
September-early October
was one of the first real indications that CFCs could react with
and destroy stratospheric ozone. The hole is not really a hole in
the ozone layer, rather a dramatic but temporary thinning of the ozone
layer above the
S. Pole and the continent of Antarctica. The ozone concentration
decreases to perhaps 30% of its normal value.
It is unusual to find clouds in the
stratosphere because there is very little water vapor there. It
gets very cold above the S. Pole in the winter though and polar
stratospheric
clouds do sometimes form (they are made from water and other
materials). This together with an
unusual wind pattern above the S. Pole in the winter are thought to
create the ozone hole when the sun returns in the spring.
The ozone destruction reactions are shown in purple above.
Cl
reacts with O3 to make ClO. This reacts with O to
produce Cl and
O2. The Cl is now available to react again with other
ozone
molecules.
In green are "interference" reactions. ClO reacts with NO2
to
make ClNO3. The Cl in this "reservoir" molecule can't
react with
any more ozone.
Now what happens above the S. Pole in the winter is that the
reservoir
molecules react on the surfaces of the polar stratospheric cloud
particles to make some kind of new compound. This reaction is
shown in orange above. The new compound HOCl accumulates in the
air during the winter. When the sun reappears in the spring, the
UV light splits off all the Cl molecules which react with ozone.
A lot of chlorine suddenly becomes available and the ozone
concentration takes a nosedive.