Wednesday Aug. 25, 2010
click here to download today's notes in a more
printer friendly format
A couple of songs Tamacun and Diablo Rojo from
Rodrigo y Gabriela before class today. They also have a very nice
version of Stairway
to
Heaven.
The Experiment signup sheets were handed out in class today. The
Experiment #1 materials should be available in class on Friday.
The only reading assignment at this point is to read through the Lecture Notes as they appear online.
We began class by reviewing some material in the Aug. 23 notes
that was either not covered or covered too quickly in class on
Monday.
The atmosphere we have today (mostly nitrogen, oxygen, water
vapor, and argon) is very different from the earth's original
atmosphere which was mostly hydrogen and helium. This
original atmosphere either escaped (the earth was hot and the gases
were moving around with enough
speed that they could overcome the pull of the earth's gravity) or was
swept into space by the solar wind (click
on the link if you are interested in learning more about the solar
wind, otherwise don't worry about it).
Most of our present atmosphere is though to have come from volcanic
eruptions.

Volcanoes didn't add any of the oxygen that is the
atmosphere.
Where did that come from?
The oxygen is thought to have first come from
photodissociation
of
water vapor and carbon dioxide by ultraviolet light (the high energy
UV light is able to split the H20 and CO2
molecules into
pieces). The O and OH react
to form O2 and H.
It is sometimes easier and clearer to show or explain a
reaction
in formulas instead of words. I don't expect you to remember
the chemical formulas in the example above. You might just
remember that the earth's original oxygen came from other gases in the
air. It's probably also good to remember that
ultraviolet light is capable of breaking molecules apart.
Once molecular oxygen (O2) begins to accumulate in the
air it can react
with atomic oxygen (O)
to form
ozone (O3). This is an example of two formulas that you
probably should remember.
Once formed, ozone in the atmosphere began to absorb ultraviolet
light and life forms could safely move from the oceans (which would
absorb UV light in the
absence of ozone) onto land. Eventually plants and photosynthesis
would become the main source of atmospheric oxygen.
Photosynthesis is a source of oxygen, it removes CO2 from the
air. Combustion is really just the opposite
of photosynthesis. We burn fossil fuels to generate energy.
Water vapor and carbon dioxide are by products. Combustion is a
source of CO2.
The
following figure is the first page in the packet of photocopied
ClassNotes.
This somewhat confusing
figure shows some of the important events in the history of the earth
and evolution of the atmosphere. The numbered points were
emphasized.
First, Point 1: the earth
is thought to be between 4.5
and
4.6 billion years old. If you want to remember the earth is a few
billion years old that is probably close enough.
The iron catastrophe was an important event (but wasn't
discussed in class). Circulation of liquid metal in the
core of
the earth gives the earth a magnetic field. The magnetic field
deflects the solar wind around the earth. Remember the solar wind
may have swept away the earth's original atmosphere.
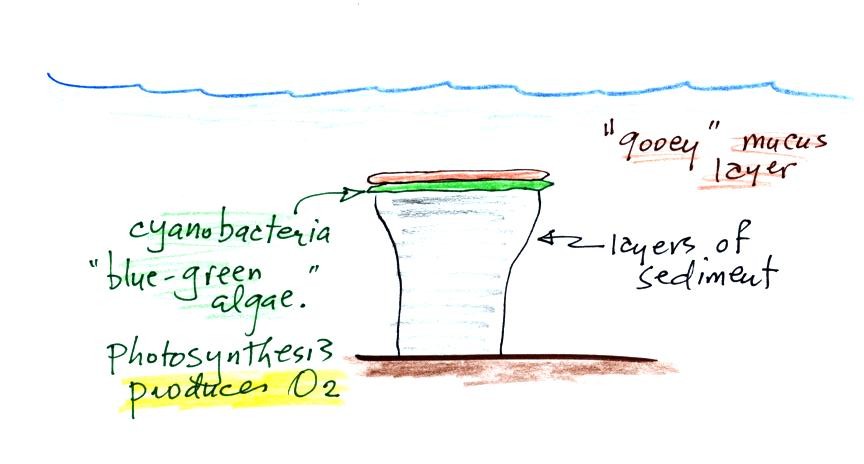
Stromatolites
(Point
2)
are
column-shaped
structures
made
up of layers of sedimentary rock, that are created by microorganisms
living at the top of the stromatolite (I've never actually seen a
stromatolite, so this is all based on photographs and written
descriptions). Fossils of the very small microbes (cyanobacteria
= blue green algae)
have been found in stromatolites as old as 2.7 B years and are some of
the earliest records of life on earth. Much older (3.5 to 3.8
B years old) stromatolites presumably also produced by microbes, but
without
microbe fossils, have been found.
We're learning about stromatolites
because the cyanobacteria were able to produce oxygen using
photosynthesis.

Living stromatolites are found
in a
few locations today. The picture above is from Coral Bay Australia, located on
the
western tip of the continent. The picture was probably taken at
low tide, the stromatolites would normally be covered with ocean water.
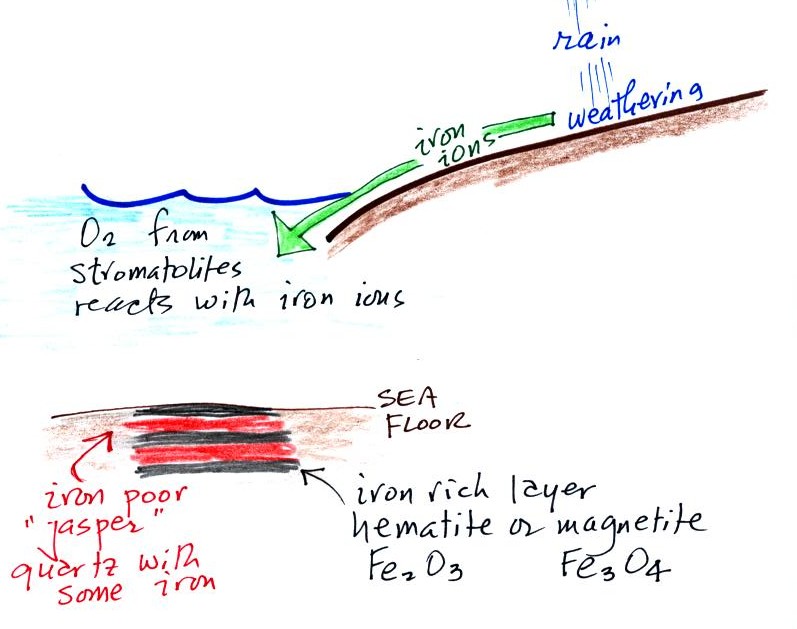
Once cyanobacteria began to produce
oxygen in ocean water, the oxygen reacted with dissolved iron (iron
ions in the figure below) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed.
Periodically the oxygen production would decrease or stop (rising
oxygen levels might have killed the cyanobacteria or seasonal changes
in incoming sunlight might have slowed the photosynthesis).
During these times of low
dissolved oxygen concentrations, layers of jasper would form on the
ocean bottom. Eventually the cyanobacteria would recover, begin
producing oxygen again, and a new layer of hematite or magnetite would
form. The rocks that resulted, containing alternating layers of
black hematite or magnetite and red layers of jasper are known as the
banded iron formation (Point 3). A couple of small
polished pieces of
banded iron rock (actually "tiger iron") were passed around
class (thanks for returning them). In addition to the red
and black layers, the tiger
iron contains yellow layers made of fibers of quartz. The
rocks are fairly heavy because they contain a lot of iron, but the most
impressive thing about them in my opinion is
their age - they are a few billion years old!
Eventually the dissolved iron in
the ocean was used up (Point 4
in the timeline figure above).
Oxygen produced by cyanobacteria no longer reacted with iron and was
free to move from the ocean into the
atmosphere. Once in the air, the oxygen could react with iron in
sediments on the earth's surface. This produced red colored
(rust colored) sedimentary rock. None of these socalled red beds
are older than
about 2 B years old. Thus it appears that a real buildup up
oxygen began around 2 B years ago. Oxygen concentrations reached levels
that are about the same as today around 500 to 600 years ago (Point 5
in the figure,).
We listed
the 5 most abundant gases in the atmosphere in class on Monday.
Several more important trace gases were added to the
list in
class today. Trace gases are gases found in low
concentrations. Low concentrations doesn't mean they aren't
important, however.
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing
gas),
chlorofluorocarbons,
and
ozone are all greenhouse gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic in the next week or two and learn more about how the
greenhouse effect actually works later in the course.
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants. We'll cover these
on Friday and early next week.
Ozone has sort of a Dr. Jeckyl and Mr. Hyde personality
(i) Ozone in the
stratosphere (a layer of the atmosphere between 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog.
We have
been discussing the composition of air. Air is mostly composed on
invisible gases. I've often thought it might be
interesting to bring in several examples of gases that you can actually
see (the gases are colored, not clear; you can't of course see the
individual gas atoms or molecules). Once I started to do some
research I found that many of these gases are very poisonous.
Here are some of the gases that you can see
Bromine
It is a heavy, volatile, mobile, dangerous reddish-brown liquid.
The
red vapour has a strong unpleasant odour and the vapour irritates the
eyes and throat. When spilled on the skin it
produces painful sores. It is a serious health hazard, and maximum
safety precautions should be taken when handling it. Since I
don't really know what maximum safety precautions entail, I won't be
bringing any bromine to class.
Chlorine
You shouldn't mix household bleach with another household cleaner
because the mixture might release chlorine gas. Chlorine
and mustard gas were used during World War I.
Iodine
Nitrogen Dioxide
Symptoms of poisoning (lung edema)
tend to appear several hours after one has inhaled a low but
potentially fatal dose. Also, low concentrations (4 ppm) will
anesthetize the nose, thus creating a potential for overexposure.
I do occasionally make nitrogen dioxide in class. It's
not
a
particularly educational demonstration but reinforces the point
that air pollutants are toxic substances.
We'll be discussing air
pollution in class starting on Friday. Air
Pollution is a serious health hazard in the US and around the
world. Click here
to download a copy of some statistics that we'll go over at
the
start
of class on Friday.