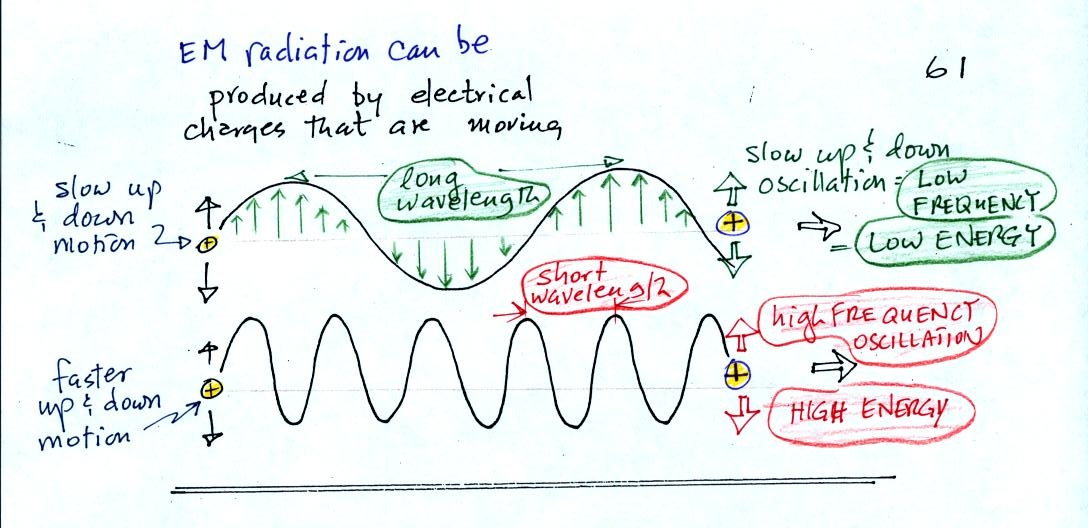
EM radiation can be created when
you cause a charge to move up and
down.
If you move a charge up and down slowly (upper left in the
figure above) you would produce long wavelength radiation that would
propagate out to the right at the speed of light. If you move the
charge up and down more rapidly you produce short wavelength radiation
that propagates at the same speed.
Once the EM radiation encounters the charges at the right side of the figure above the EM radiation causes those charges to oscillate up and down. In the case of the long wavelength radiation the charge at right oscillates slowly. This is low frequency and low energy motion. The short wavelength causes the charge at right to oscillate more rapidly - high frequency and high energy.
These three characteristics: long wavelength / low frequency / low energy go together. So do short wavelength / high frequency / high energy. Note that the two different types of radiation both propagate at the same speed.
The following figure illustrates how energy can be transported from one place to another (even through empty space) in the form of electromagnetic (EM) radiation.
Once the EM radiation encounters the charges at the right side of the figure above the EM radiation causes those charges to oscillate up and down. In the case of the long wavelength radiation the charge at right oscillates slowly. This is low frequency and low energy motion. The short wavelength causes the charge at right to oscillate more rapidly - high frequency and high energy.
These three characteristics: long wavelength / low frequency / low energy go together. So do short wavelength / high frequency / high energy. Note that the two different types of radiation both propagate at the same speed.
The following figure illustrates how energy can be transported from one place to another (even through empty space) in the form of electromagnetic (EM) radiation.
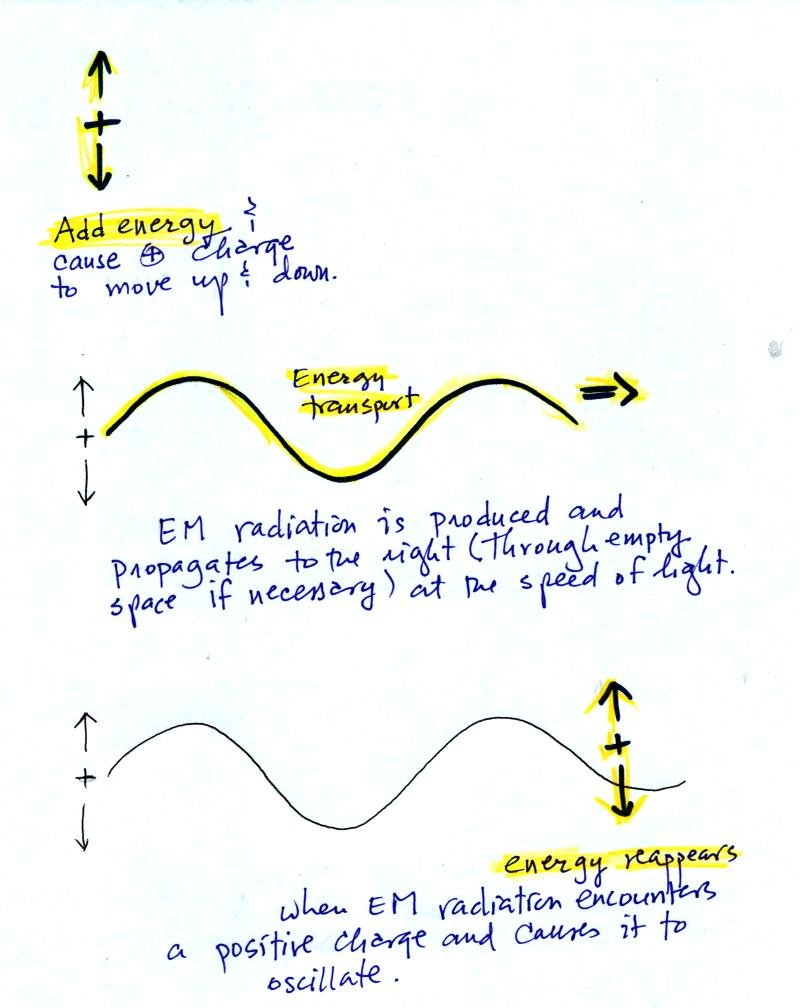
In the middle
figure, the EM
radiation then travels out
to the
right (it could be through empty space or through something like the
atmosphere).
Once
the EM radiation encounters an electrical charge at another location
(bottom right),
the energy reappears as the radiation causes the charge to move.
Energy
has been transported from left to right.
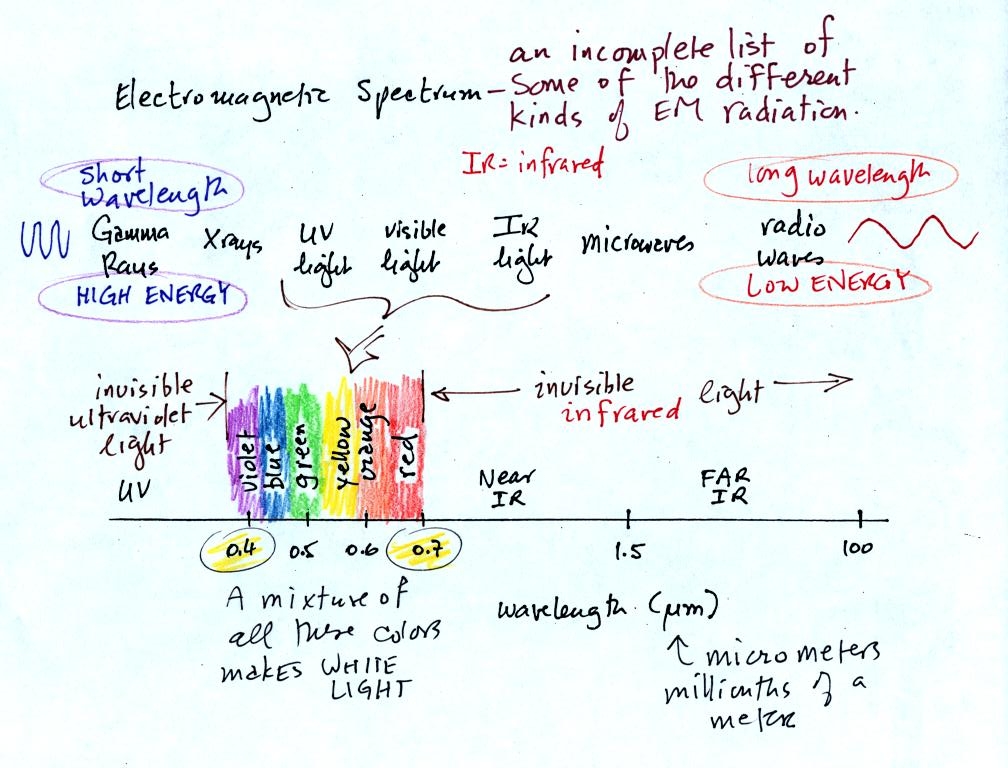
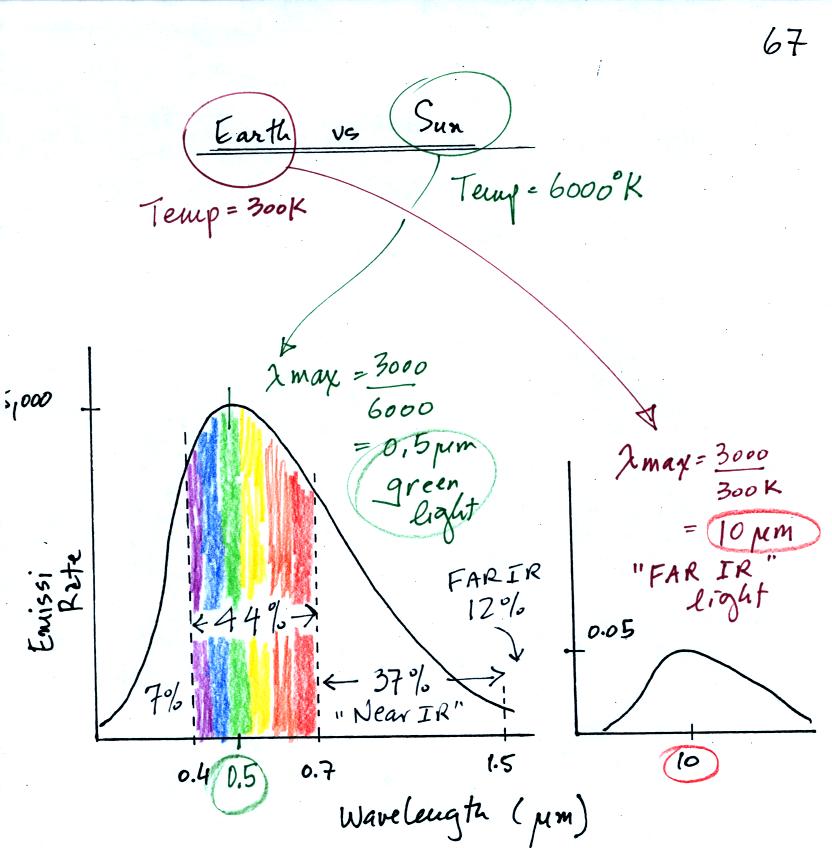
This is really just a partial list of some of the different types of EM radiation. In the top list, shortwave length and high energy forms of EM radiation are on the left (gamma rays and X-rays for example). Microwaves and radiowaves are longer wavelength, lower energy forms of EM radiation.
We will mostly be concerned with just ultraviolet light (UV), visible light (VIS), and infrared light (IR). Note the micrometer (millionths of a meter) units used for wavelength for these kinds of light. The visible portion of the spectrum falls between 0.4 and 0.7 micrometers. UV and IR light are both invisible. All of the vivid colors shown above are just EM radiation with slightly different wavelengths. When you see all of these colors mixed together, you see white light.
Here are some rules governing the emission of electromagnetic radiation:
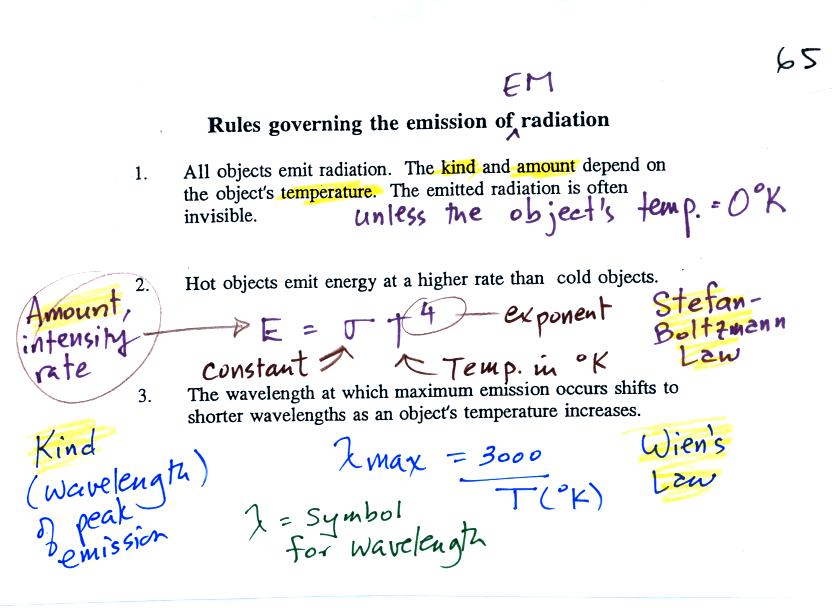
1.
Unless an object
is very cold (0
K) it will emit EM
radiation. Everything in the classroom: the people, the
furniture, the walls and the floor, even the air, are emitting EM
radiation. Often
this radiation
will be invisible so that we can't see it and weak enough that we can't
feel it (or perhaps because it is always there we've grown accustomed
to it and ignore it). Both the amount and kind (wavelength) of
the emitted
radiation depend on the object's temperature.
2.
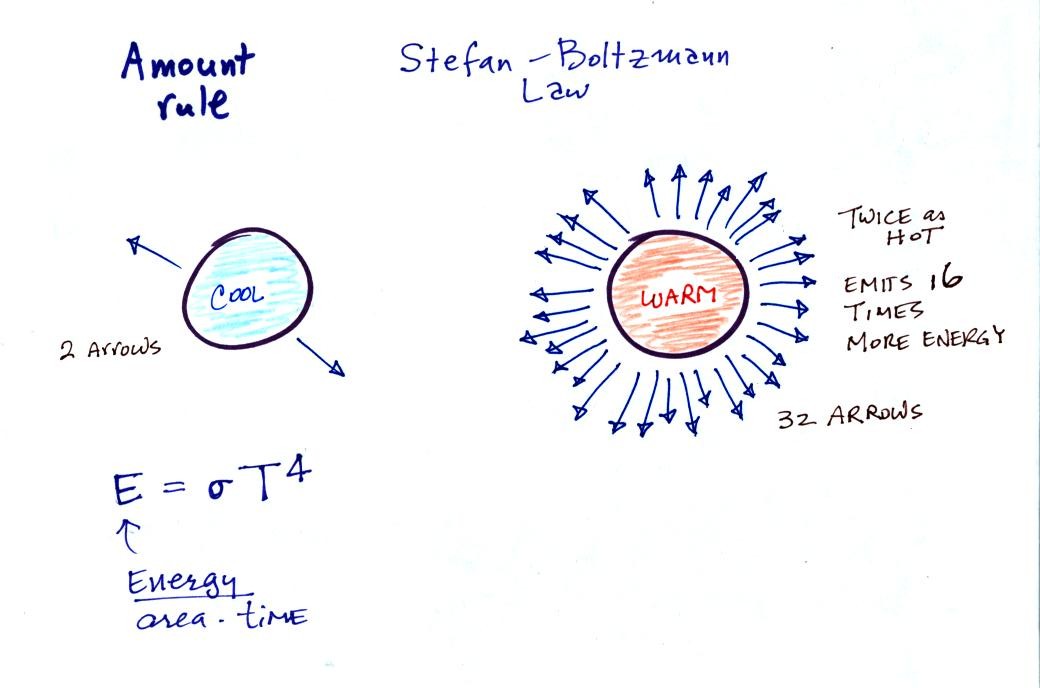
The second rule
allows you to
determine the amount of EM radiation (radiant energy) an object will
emit. Don't worry about the units,
you can think of this as amount, or rate, or intensity.
Don't worry about σ either, it is just a
constant. The amount depends on temperature to
the fourth
power. If the temperature of an object doubles the amount of
energy emitted will increase by a factor of 2 to the 4th power
(that's 2 x 2 x 2 x 2 = 16). A hot object just doesn't emit a
little more energy than a
cold object it emits a lot more energy than a cold object. This
is illustrated in the following figure:
3.
The third rule
tells you something
about the kind of radiation emitted
by an object. We will see that objects usually emit radiation at
many different wavelengths but not in equal amounts. Objects emit
more of one particular wavelength than any of the others. This is
called lambda max (lambda is the greek character used to
represent wavelength) and is called the wavelength of maximum
emission. The third rule allows
you to calculate "lambda max." The tendency for warm objects to
emit radiation at shorter wavelengths is shown below.
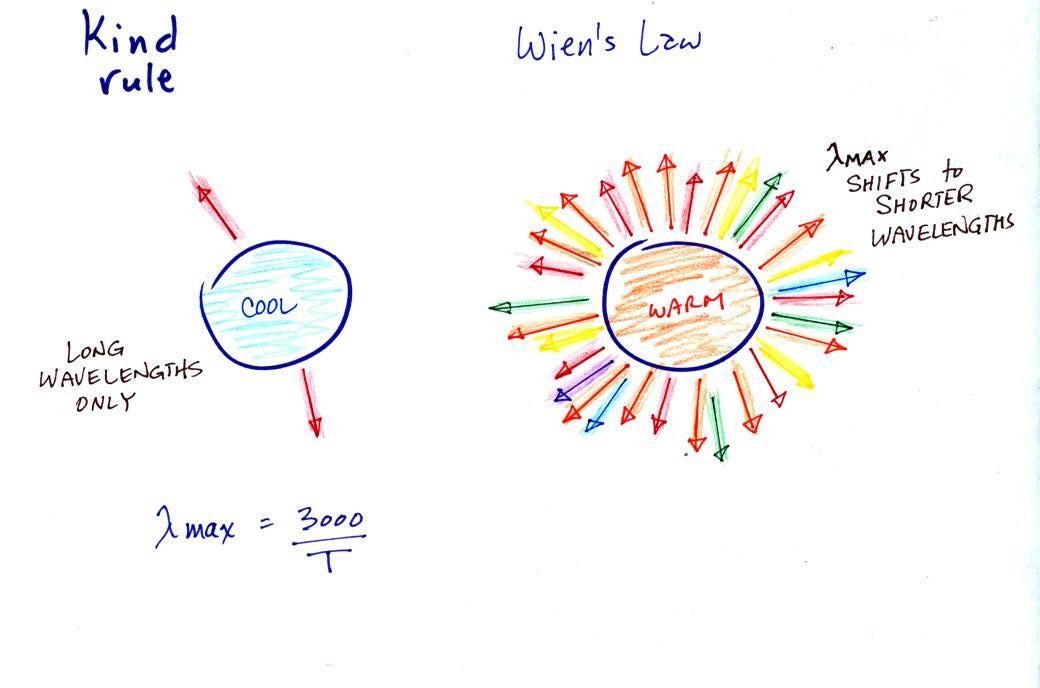
The cool object is shown emitting
red light. It could be visible light or IR light. The
warmer object will also emit IR and red light but also shorter
wavelengths such as yellow, green, blue, and violet (maybe even some UV
if it's warm enough).
If the cool object is emitting visible light it would appear red. Because the warmer object is emitting a mix of visible colors, it would probably appear white.
The graphs at the bottom of p. 65 in the photocopied ClassNotes also help to illustrate the Stefan-Boltzmann law and Wien's laws.
If the cool object is emitting visible light it would appear red. Because the warmer object is emitting a mix of visible colors, it would probably appear white.
The graphs at the bottom of p. 65 in the photocopied ClassNotes also help to illustrate the Stefan-Boltzmann law and Wien's laws.
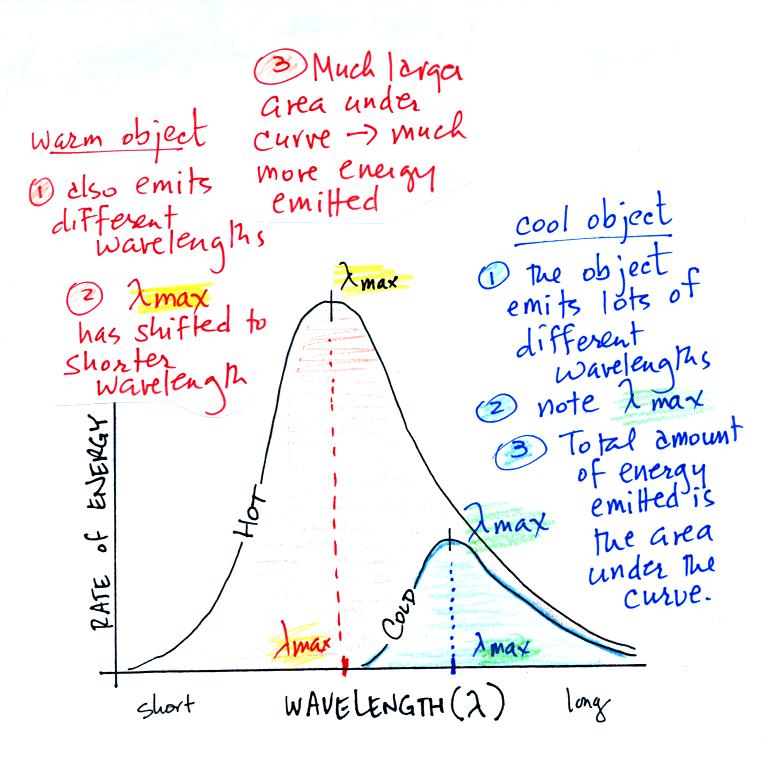
1.
Notice first
that both and warm
and
the cold objects emit radiation
over a range of wavelengths (the curves above are like quiz scores, not
everyone gets the same score, there is a distribution of grades)
2.
Lambda max has
shifted toward
shorter wavelengths for the warmer
object. This is Wien's law in action. The warmer object is
emitting lots of types of short wavelength radiation that the colder
object
doesn't emit.
3.
The area under
the warm object
curve is much bigger than the area under
the cold object curve. The area under the curve is a measure of
the total radiant energy emitted by the object. This illustrates
the fact that the warmer object emits a lot more radiant energy than
the colder object.
An ordinary 200 W tungsten bulb connected to a dimmer switch can be used to demonstrate these rules (see p. 66 in the photocopied ClassNotes). We'll be seeing the EM radiation emitted by the bulb filament.
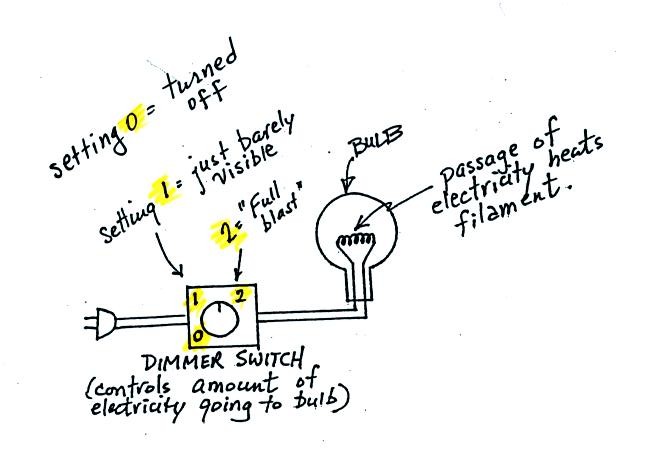
The graph at the bottom of p. 66 has been split up into 3 parts and redrawn for improved clarity.
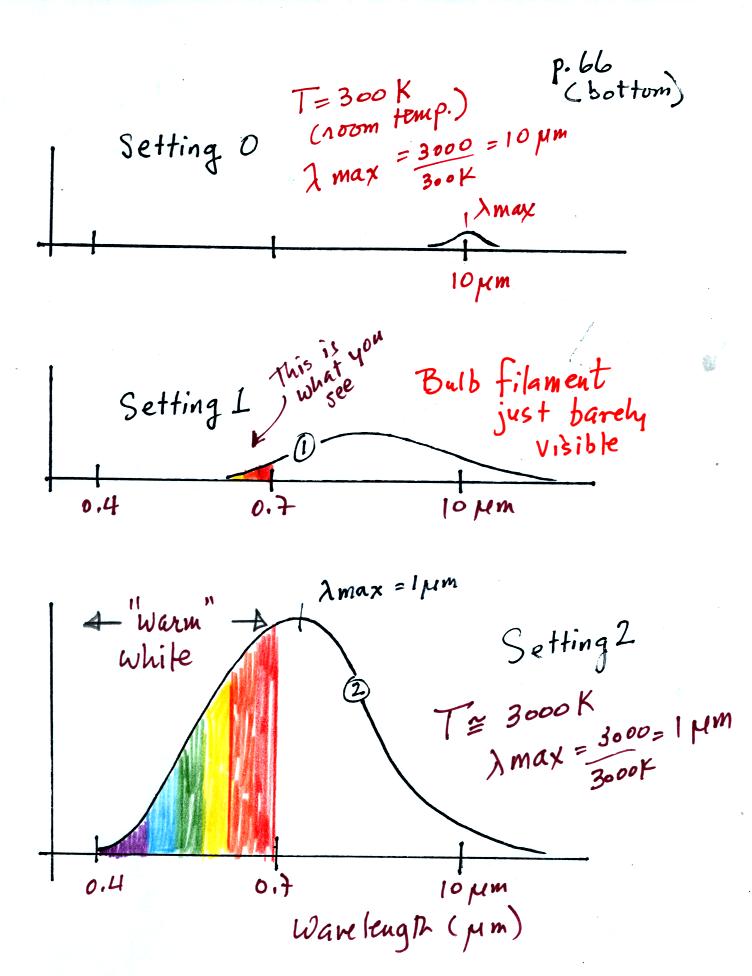
We start with the bulb turned off (Setting 0). The filament will be at room temperature which we will assume is around 300 K (remember that is a reasonable and easy to remember value for the average temperature of the earth's surface). The bulb will be emitting radiation, it's shown on the top graph above. The radiation is very weak so we can't feel it. The wavelength of peak emission is 10 micrometers which is long wavelength, far IR, radiation so we can't see it.
Next we use the dimmer switch to just barely turn the bulb on (the temperature of the filament is now about 900 K). The bulb wasn't very bright at all and had an orange color. This is curve 1, the middle figure. Note the far left end of the emission curve has moved left of the 0.7 micrometer mark - into the visible portion of the spectrum. That is what you are able to see, just the small fraction of the radiation emitted by the bulb that is visible light (but just long wavelength red and orange light). Most of the radiation emitted by the bulb is to the right of the 0.7 micrometer mark and is invisible IR radiation (it is strong enough now that you could feel it if you put your hand next to the bulb).
Finally we turn on the bulb completely (it was a 200 Watt bulb so it got pretty bright). The filament temperature is now about 3000K. The bulb is emitting a lot more visible light, all the colors, though not all in equal amounts. The mixture of the colors produces a "warm white" light. It is warm because it is a mixture that contains a lot more red, orange, and yellow than blue, green, and violet light. It is interesting that most of the radiation emitted by the bulb is still in the IR portion of the spectrum (lambda max is 1 micrometer). This is invisible light. A tungsten bulb like this is not especially efficient, at least not as a source of visible light.
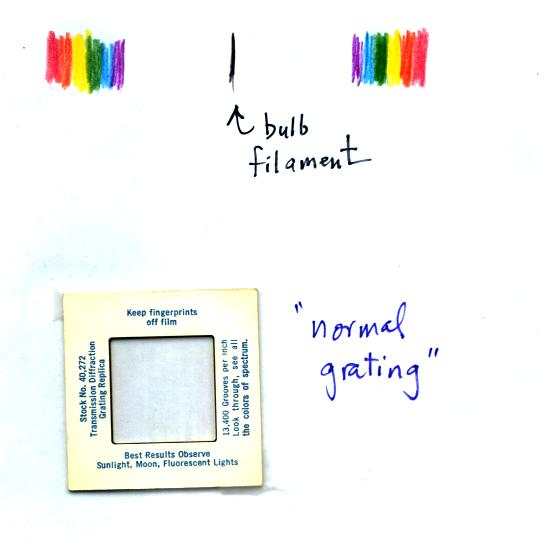
You were able to use one of the diffraction gratings handed out in class to separate the white light produced by the bulb into its separate colors.
When you looked at the bright white bulb filament through one of the diffraction gratings the colors were smeared out to the right and left as shown below.
Some of the gratings handed out in class behaved a little
differently
and spread out the colors horizontally, vertically, and diagonally.
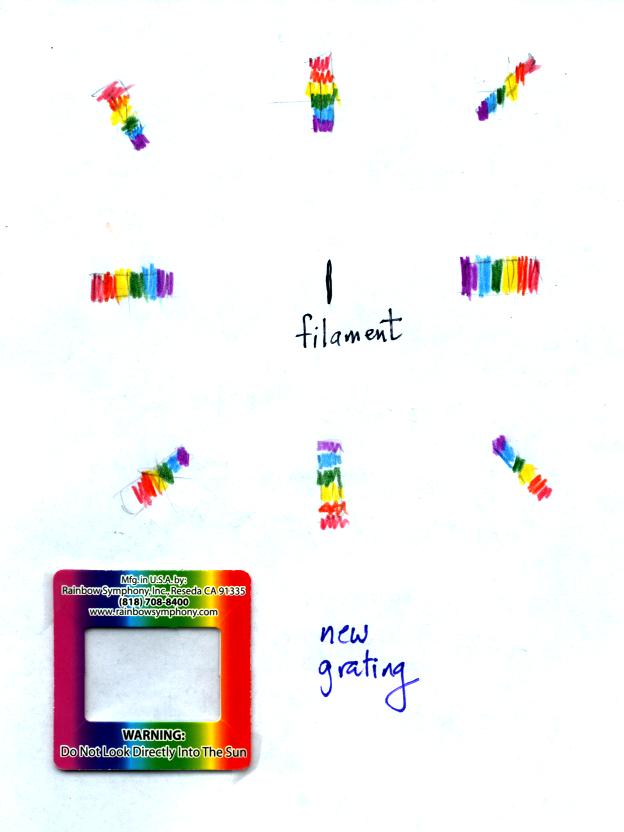
We had time for one more page of
notes.