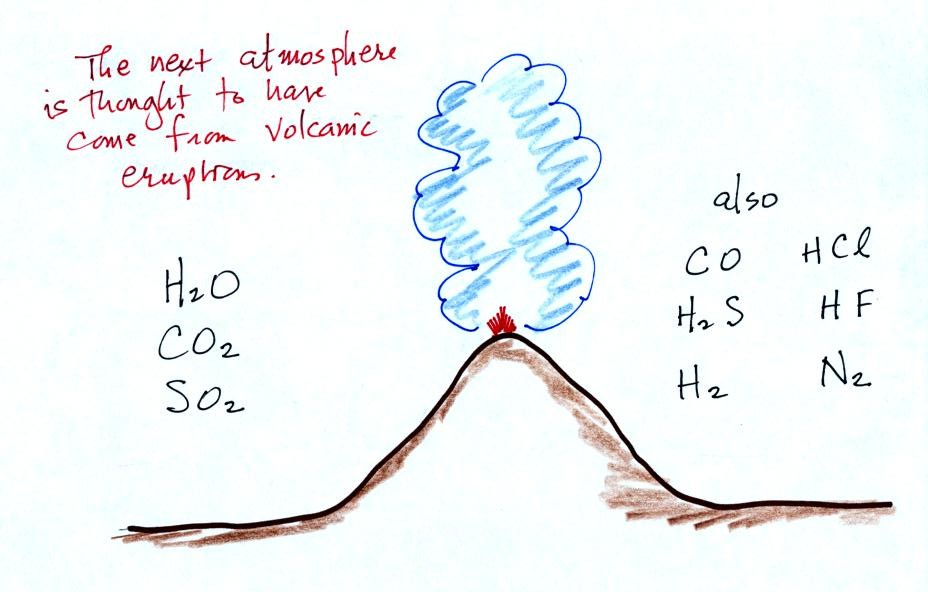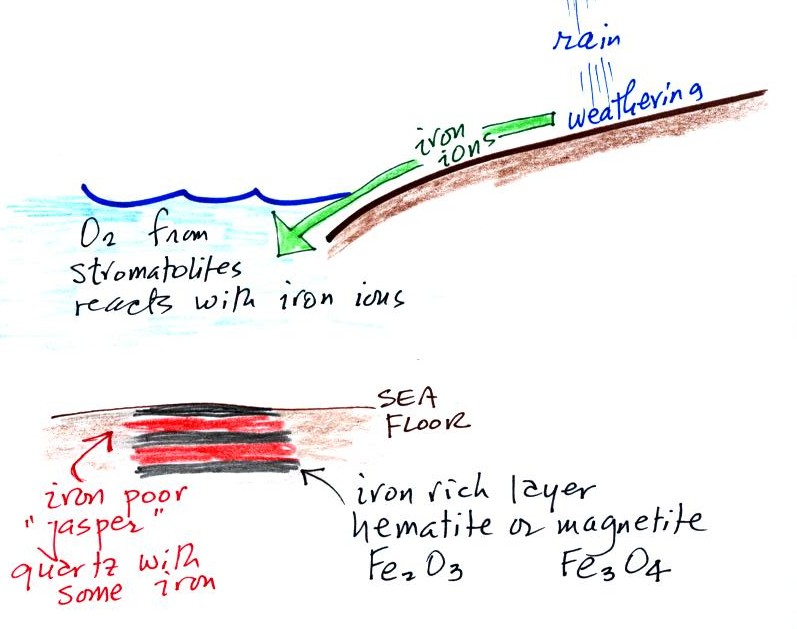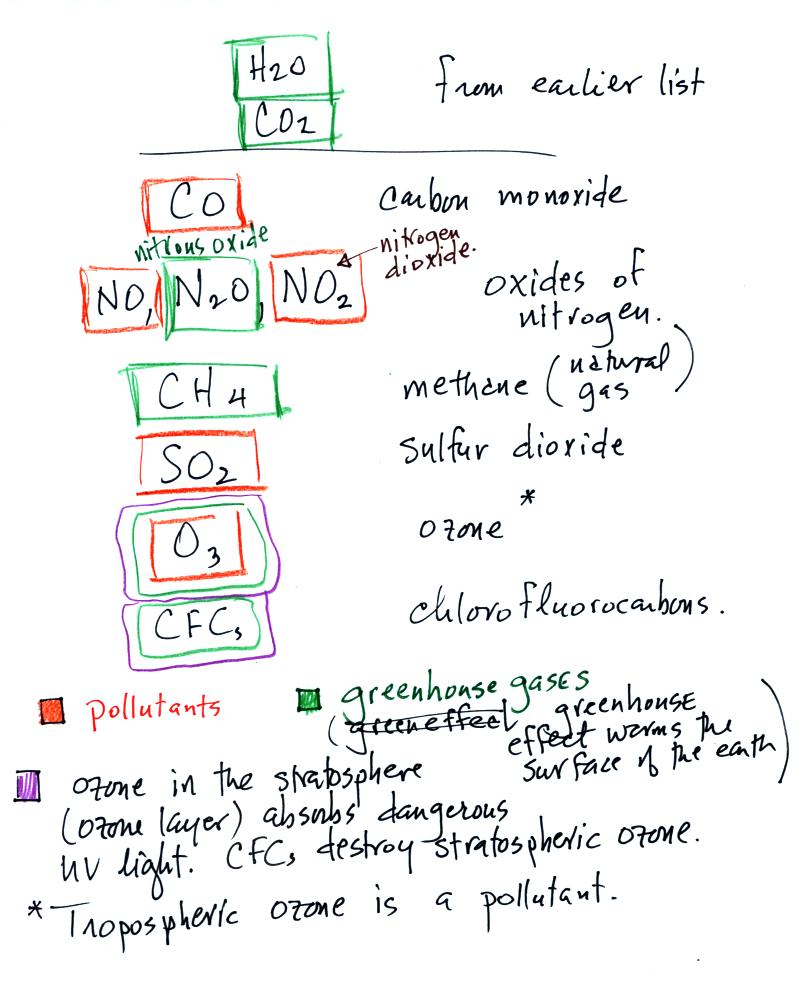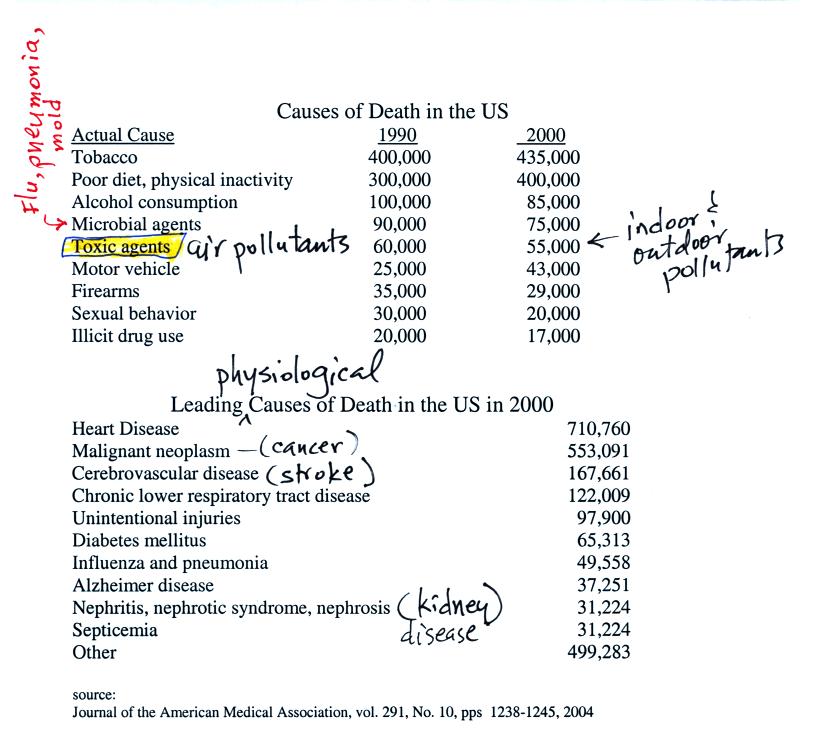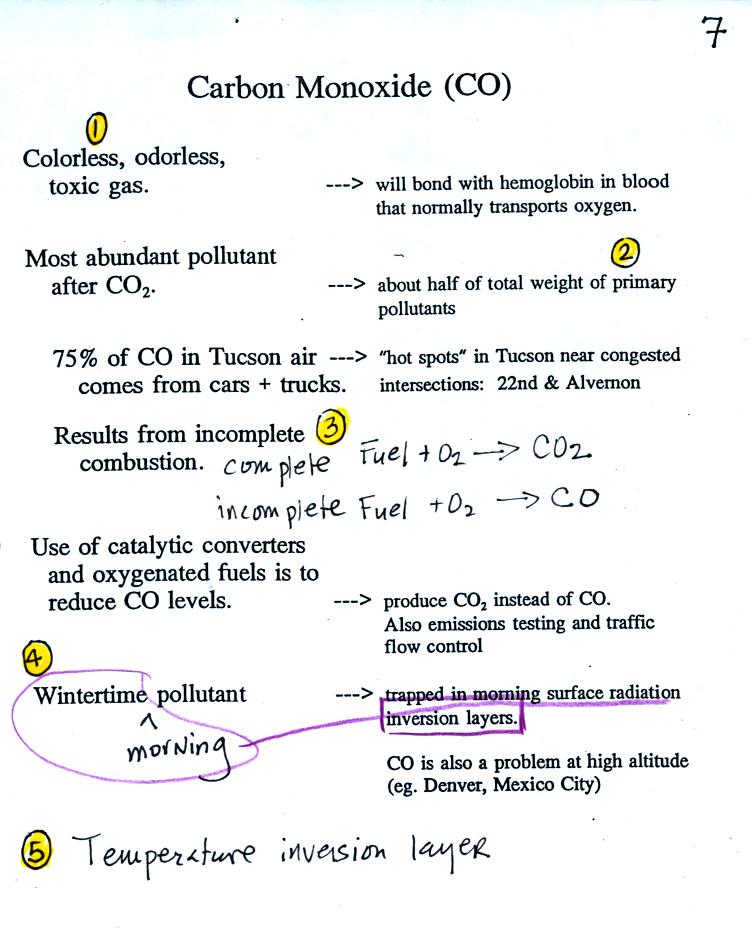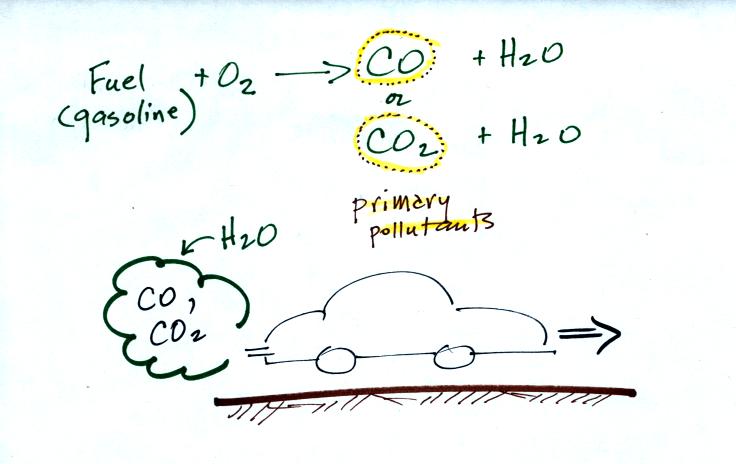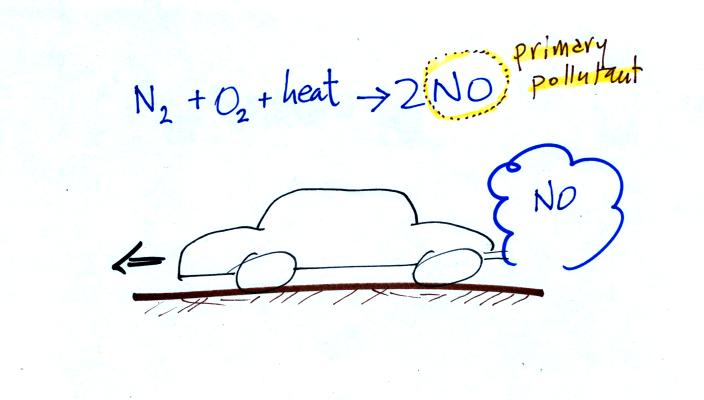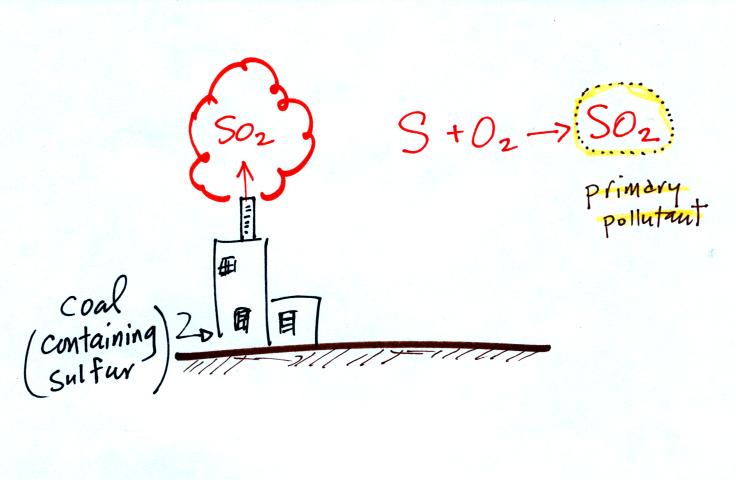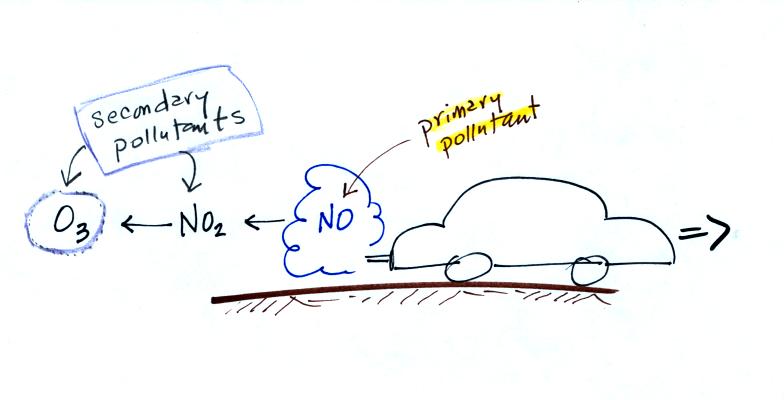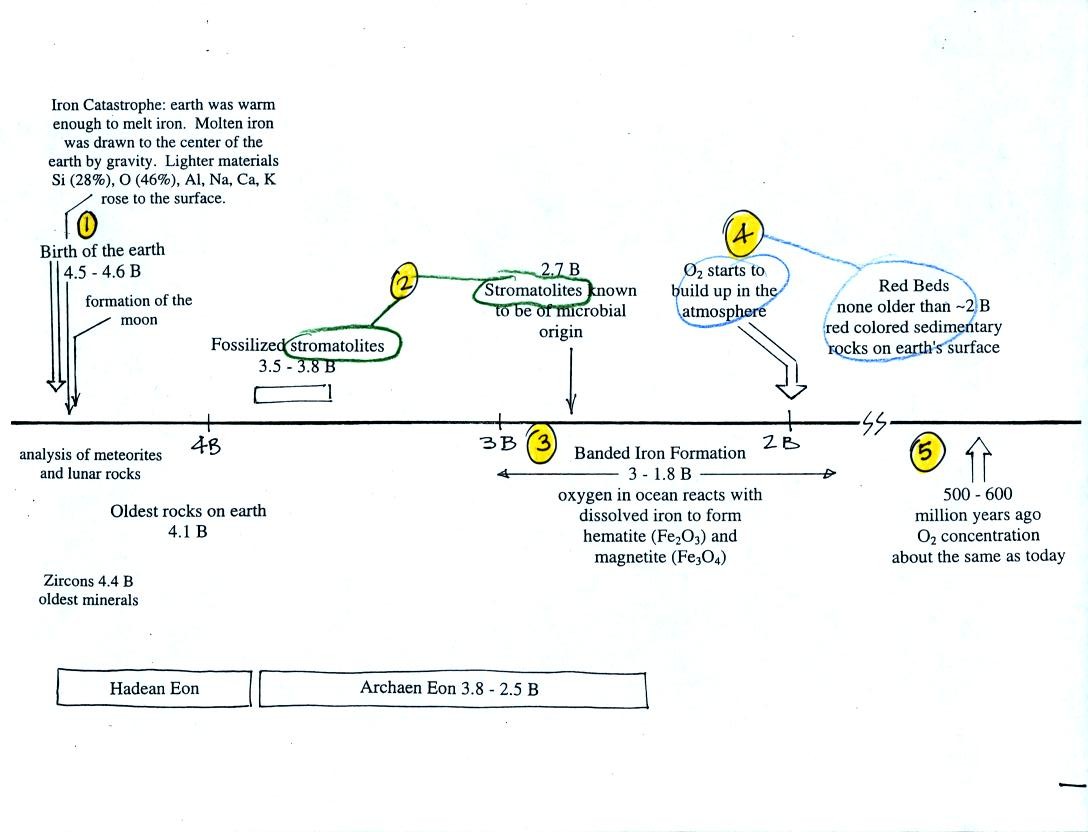
This confusing
figure shows some of the important events in the history of the earth
and evolution of the atmosphere. The numbered points were
emphasized.
First, Point 1: the earth
is thought to be between 4.5
and
4.6 billion years old. If you want to remember the earth is a few
billion years old that is probably close enough.
The iron catastrophe was an important event (but wasn't
discussed in class). Circulation of liquid metal in the
core of
the earth gives the earth a magnetic field. The magnetic field
deflects the solar wind around the earth. Remember the solar wind
may have swept away the earth's original atmosphere.
Stromatolites
(Point
2)
are
column-shaped
structures
made
up of layers of sedimentary rock, that are created by microorganisms
living at the top of the stromatolite (I've never actually seen a
stromatolite, so this is all based on photographs and written
descriptions). Fossils of the very small microbes (cyanobacteria
= blue green algae)
have been found in stromatolites as old as 2.7 B years and are some of
the earliest records of life on earth. Much older (3.5 to 3.8
B years old) stromatolites presumably also produced by microbes, but
without
microbe fossils, have been found.