Tuesday Sept. 7, 2010
click here to download today's notes in
a more printer friendly format
The first day after a long 3-day weekend seemed like a good
place for a couple
of songs from the Flobots
("Handlebars" and "By the Time You Get This Message").
All of the names on the Report Signup sheets should now be on the Online Lists. If you name isn't
there and you think it should be, let me know. Sorry about any
misspelled names.
The Practice Quiz is Thursday this week (during the 2nd half or so
of the class period) There'll be a Practice Quiz review this
afternoon from 4-5 pm in Haury 129 (aka Anthropology
129) and Wednesday afternoon at the same time in Soc. Sci. 22.
Now that we have finished the section on
air pollutants, here's a list of the key
points for each of the pollutants that we covered.
carbon monoxide
(CO)
colorless, odorless
primary pollutant
incomplete combustion
winter, morning pollutant
temperature inversion layer
|
tropospheric
ozone (O3)
secondary pollutant
summer, afternoon pollutant
Los Angeles - type (photochemical smog)
|
sulfur dioxide
(SO2)
1st pollutant
London - type smog
acid rain
|
particulate
matter (PM)
health hazard
affects visibility |
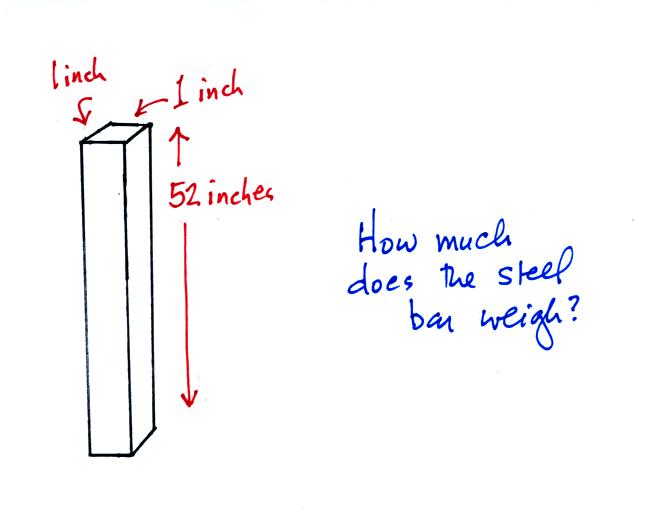
An iron bar was passed around at the
beginning of class. You were supposed to guess how much it
weighed.
We came back to this later in the period.
Bottles containing approximately equal volumes of
water and mercury were passed around in class (thanks for being careful
with the mercury). There is a lot more mass in the bottle of
mercury than in the bottle of water. Because it has more mass the
bottle of mercury also weighs more than the bottle of water (that's
something you can feel). Mercury is much denser than water.
Before we can learn about
atmospheric pressure, we
need to review
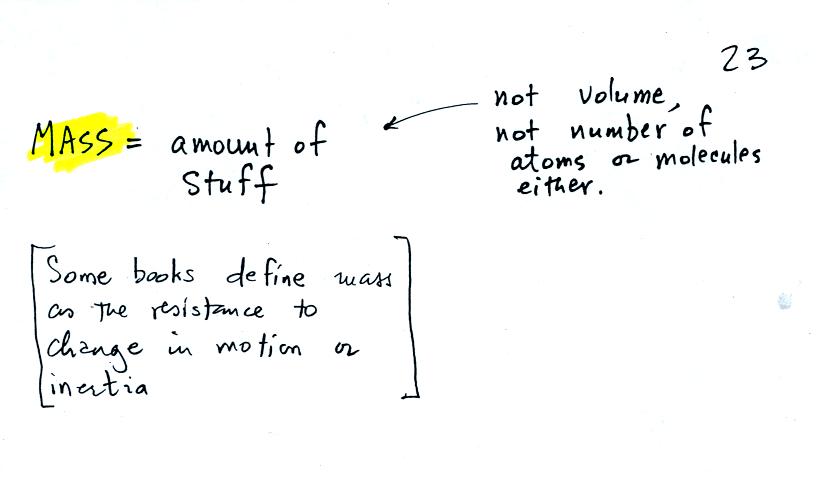
the terms mass and weight. In some textbooks you'll find mass
defined as "amount of stuff" or "amount of a particular
material." Other books will define mass as
inertia or as resistance to change in motion (this comes from Newton's
2nd law of motion, we'll cover that later in the semester). The
next picture
illustrates both these definitions.
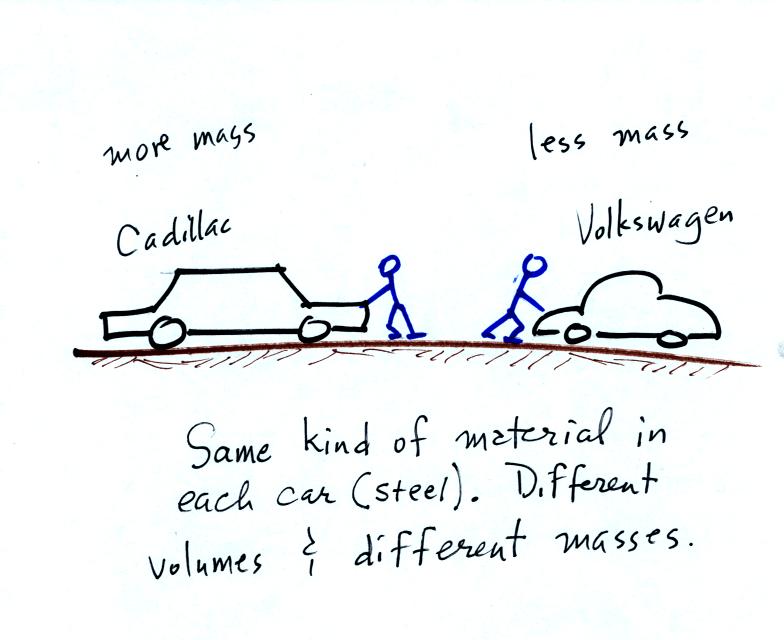
A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac is also much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down than the
Volkswagen once it is
moving).
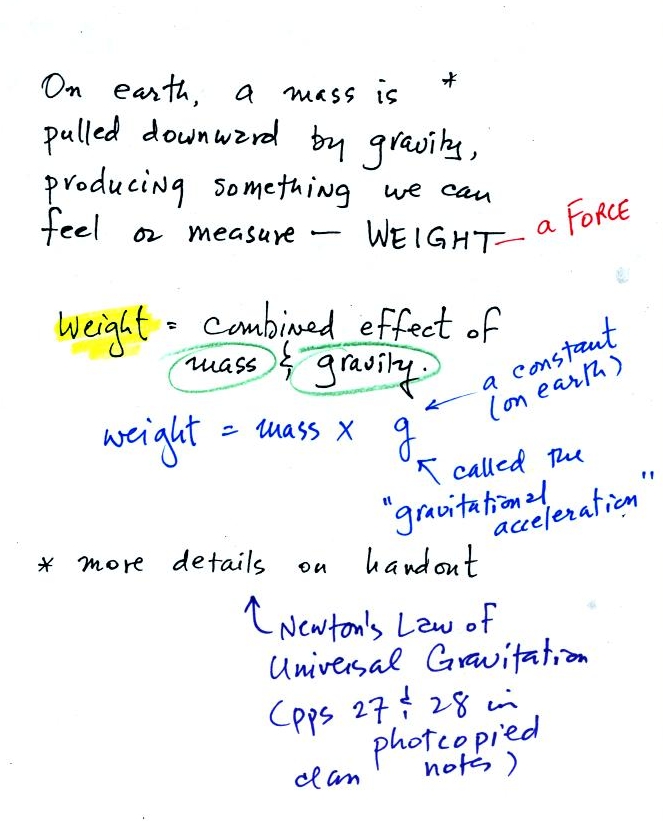
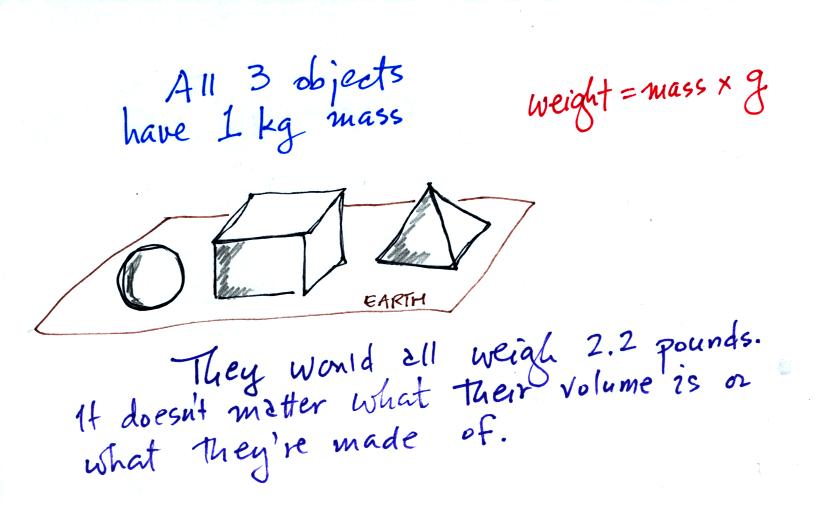
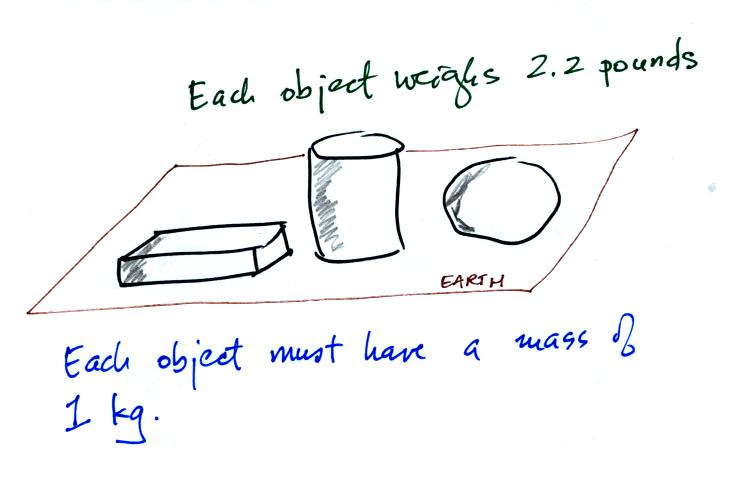
On the earth where the pull of
gravity never changes, any three objects
that all have the same mass
(even if they had different volumes and were made of different
materials) would always have the same weight. Conversely:
When
gravity
is
always
the
same,
three
objects
with
the
same
weight
would also have the same mass.
The
difference
between
mass
and
weight
is
clearer
(perhaps)
if you
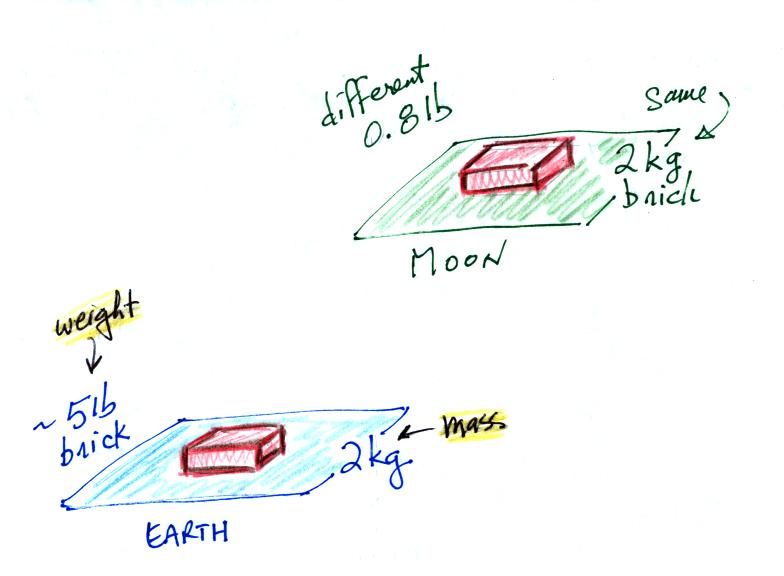
compare the situation on the earth and on the moon.
On the earth a brick with a mass of about 2 kg weighs about 5
pounds. If you carried the brick to the moon it would have the
same mass. But gravity on the moon is weaker than on the
earth. Objects on the moon weigh less than on the earth.
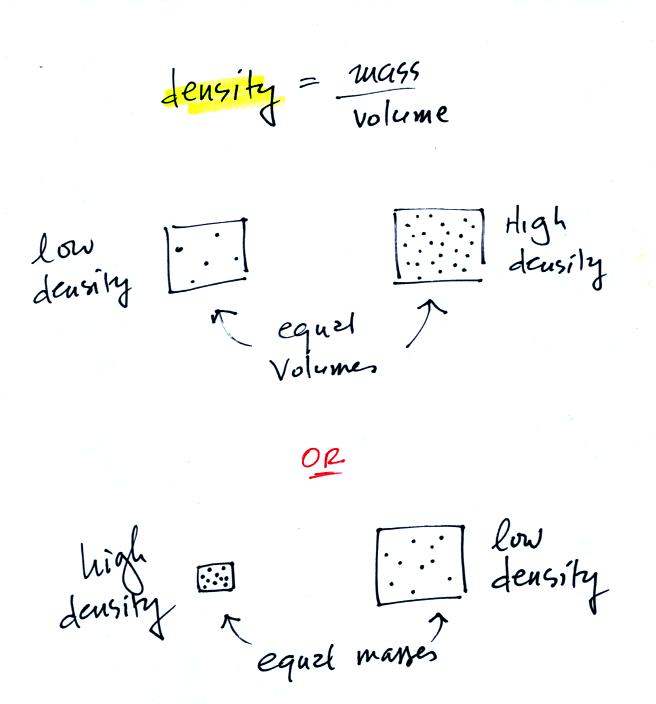
In
the first example there is more mass (more dots) in the right box than
in the left box. Since the two volumes are equal the box at right
has higher density. Equal masses are squeezed into different
volumes in the bottom example. The box with smaller volume has
higher density.
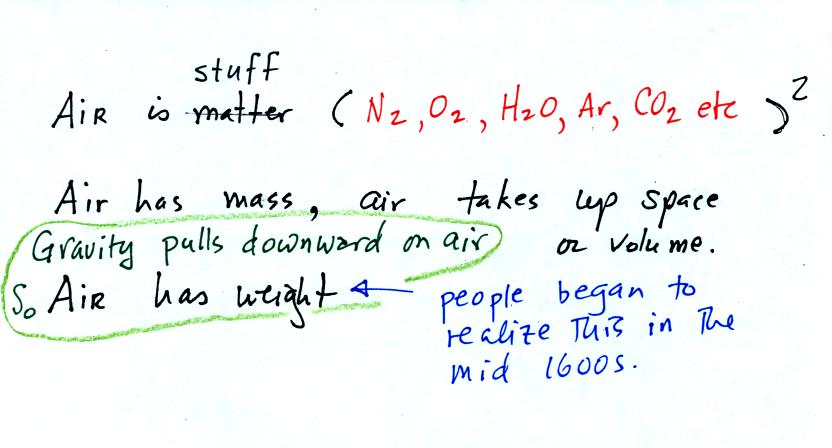
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment
to
prove that air has weight. The experiment wasn't mentioned
in class.
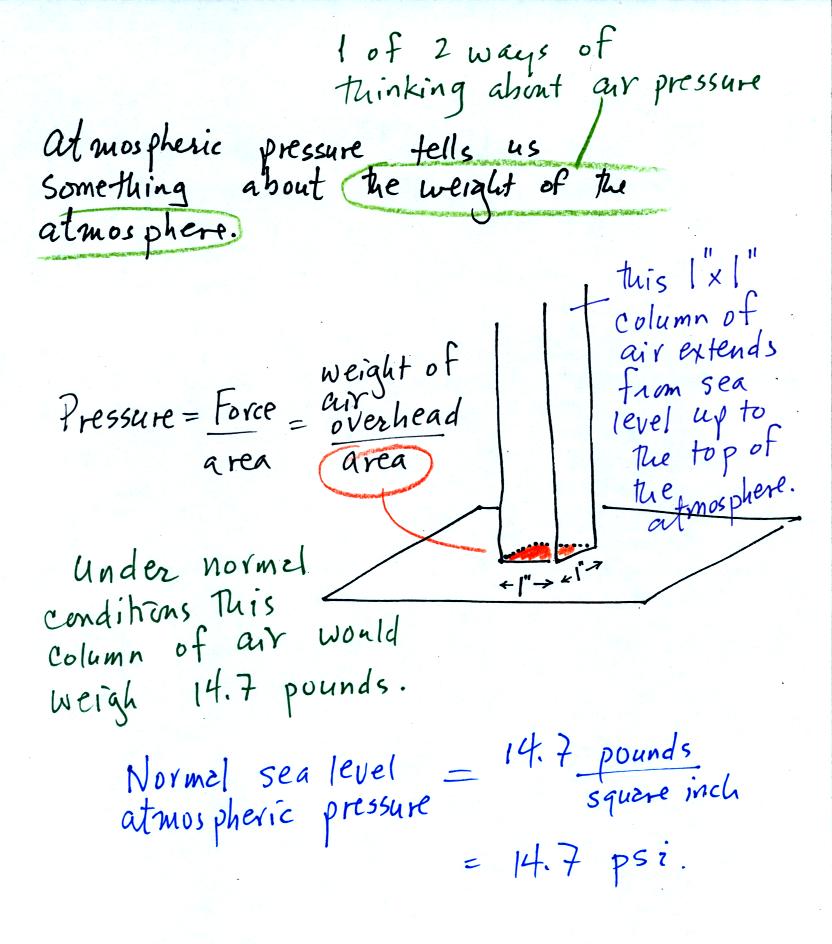
Pressure is defined as force divided by area. Air
pressure is the
weight
of the atmosphere overhead divided by the area the air is resting
on.
Atmospheric pressure is
determined by and tells you something about the weight of the air
overhead. This is one way, a sort of large scale representation,
of understanding air pressure.
Under normal conditions a 1 inch by 1 inch column of air
stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your
car
or
bike
tires
with
air).
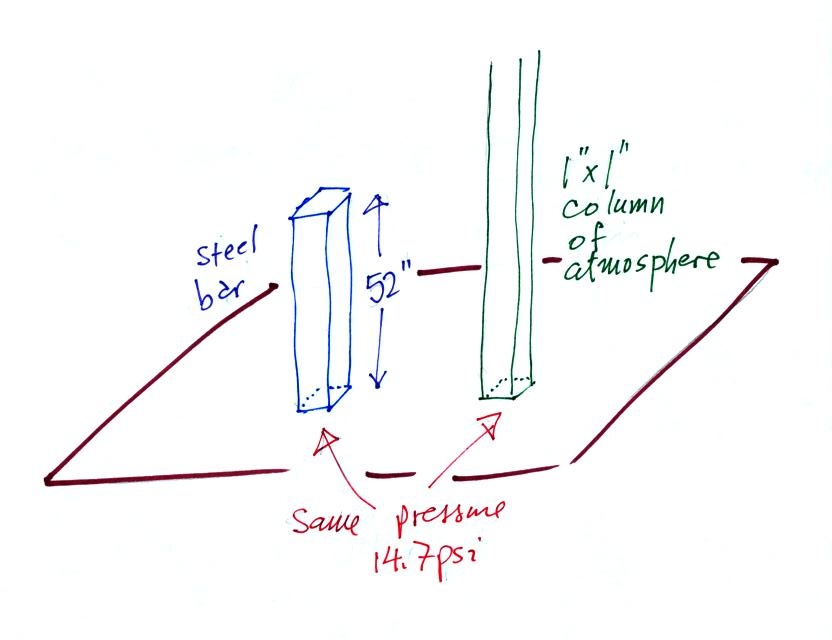
Now here's
where the steel bar
comes in. The steel bar also weighs exactly 14.7 pounds.
Steel is a lot denser
than air, so a steel bar only needs to be
52 inches tall to have the same weight as an air column that is 100
miles or more tall. If a mercury bar had been used it would only
have to be about 30 inches long.
14.7 psi is one weigh of expressing average sea level
pressure. Here are average sea level pressure values in different
units.
Typical sea level
pressure is 14.7 psi or about 1000 millibars
(the
units used by meterologists and the units that we will use in this
class most of the time) or about 30 inches of mercury (refers to
the reading on a mercury barometer). If you ever find
yourself in France needing to fill your
automobile tires with air (I lived in France for a while and owned
a Peugeot
404)
remember that the air compressor scale is
probably calibrated in bars. 2 bars of pressure would be
equivalent to 30 psi.
Pressure
at sea level is determined by the weight of the air overhead.
What happens to pressure as you move
upward in the atmosphere. We can use a pile of bricks to help
answer this question. I use bricks because you can see
them. You can think of the bricks representing layers of air in
the atmosphere.

The atmosphere is really no different. Pressure at any level is
determined by
the weight of the air still overhead. Pressure decreases with
increasing altitude because there is less and less air remaining
overhead. The figure is a more
carefully drawn version of what was done in class.
At sea
level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all (100%) of the air in the atmosphere.
Some parts of Tucson, at Point 2, are 3000
feet above sea level (most
of the valley is a little lower than that around 2500 feet). At
3000 ft. about 10%
of the
air is
below, 90% is still overhead. It is the weight of the 90% that is
still above that determines the atmospheric pressure in Tucson.
If 100% of the atmosphere produces a pressure of 1000 mb, then 90% will
produce a pressure of 900 mb.
Pressure is typically about 700 mb at the
summit of Mt. Lemmon (9000
ft. altitude at Point 3) and
70% of the atmosphere is overhead..
Pressure decreases rapidly with increasing
altitude. We will find that pressure changes more slowly if you
move horizontally. Pressure changes about 1 mb for every 10
meters of elevation change. Pressure changes much more slowly
normally if you move horizontally: about 1 mb in 100 km. Still
the small horizontal changes are what
cause the
wind to blow and what cause storms to form.
Point 4 shows
a
submarine
at
a
depth
of
about
33 ft. or so. The pressure
there is determined by the weight of the air and the weight of the
water overhead. Water is much denser and much heavier than
air. At 33 ft., the pressure is already twice what it would be at
the surface of the ocean (2000 mb instead of 1000 mb).
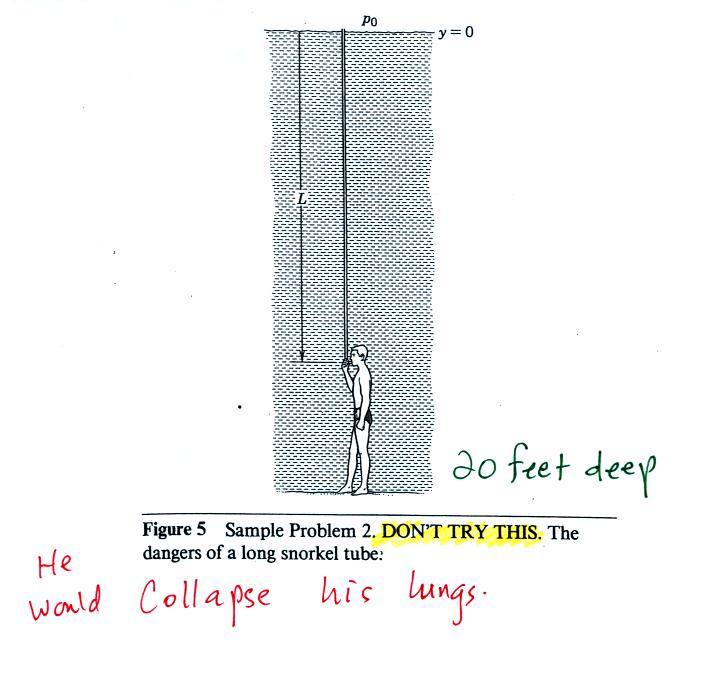
The person in the picture below (not shown in class)
is
20
feet
underwater.
At
that
depth there is a pretty large pressure
pushing against his body
from the surrounding water. The top of the snorkel is exposed to
the much lower air pressure at the top of the pool. If the
swimmer puts his mouth on the snorkel the pressure at the bottom of the
pull would collapse his lungs.
We took a
bit of a detour at this point.
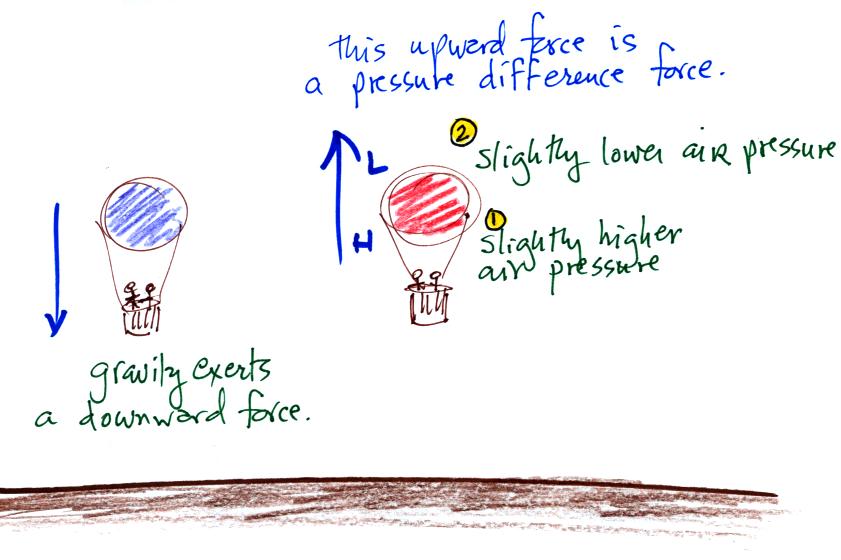
Hot air balloons can rise or can sink. Most everyone in the
classroom knew that gravity is what causes a balloon (or another
object) to fall. Not very many people know that an upward
pressure force is what can cause a balloon to float upward.
Pressure decreases with increasing altitude. That means that the
air pressure pushing against the bottom of a balloon is a little bit
stronger than the force pushing against the top of the balloon.
There isn't much of a difference in the strength of the two forces but
there is a difference. And this force points upward from high
toward lower pressure.
The two forces are always opposing each other. When the air in
the balloon is cold and dense the balloon sinks. When the air is
hot and has low density the balloon rises.
We'll come back to this topic in a week or so, don't worry if you
aren't understanding it fully right now.
Air is
compressible, so a pile of mattresses (clean
mattresses not the disgusting things you sometimes see at the curb in
front of someone's house) might be a more realistic representation of
layers of air in the atmosphere. We can use mattresses to
understand how air density changes with increasing altitude.
Four mattresses are stacked on top of each other. Mattresses
are reasonably heavy, the mattress at the
bottom of the
pile is compressed the most by the weight of all the mattresses
above. This is shown at right. The mattresses higher up
aren't squished as much because
their
is less weight remaining above. The same is true with layers of
air in the atmosphere.
Here's a slightly clearer version of the figure drawn in class
There's a lot of information in
this figure. It is worth
spending a minute or two looking at it and thinking about it.
1. You can first notice and remember that pressure
decreases
with increasing altitude. 1000 mb at the bottom decreases to 700
mb at the top of the picture.
Each layer of air contain the same amount (mass) of air.
This is a fairly subtle point. You
can
tell because the pressure decrease as you move upward through each
layer is the same (100 mb). Pressure depends on weight. So
if the pressure change is the same everytime you move up one layer, the
weights of each of the layers must be equal. Each of the layers
must contain the same amount (mass) of air. Each layer contains
10% of the air in
the atmosphere.
2. The densest air is found in the bottom
layer. Each layer has the same amount of air
(same mass). The bottom layer is compressed the most so it has
the
smallest volume. Mass/( small volume)
gives a high density. The top layer has the same amount of air
but about twice the volume. It therefore has a lower density.
3. The rate of pressure change with altitude depends on air
density. The most rapid rate of pressure decrease with increasing
altitude is in the densest air at the bottom of the picture.
This is where class ended today. But I have added one more figure
that explains why the rate of pressure change as you move or down in
the atmosphere depends on air density. The following figure
wasn't shown in class.

There is a lot going on here.
Point 1 - Notice there is a 100 mb drop in pressure in both air
layers. In order for this to be true both layers must weigh the
same. In order for both layers to have the same weight they must
contain the same amount of air, they have the same mass.
Point 2a - The pressure decreases 100 mb in a relatively short
distance. This produces a relatively rapid rate of pressure
decrease with increasing altitude.
Point 2b - The pressure also decreases 100 mb but in a longer
distance. Pressure is decreasing at a slower rate in this layer.
Point 3 - The air in the left layer is denser than the air
in
the right layer. The same amount (mass) of air is squeezed into a
thinner layer, a smaller volume, in the left layer. This results
in relatively high density air.
The fact that the rate of pressure decrease with increasing
altitude depends on air density is a fairly subtle but important
concept. This concept
will come up 2 or 3 more times later in the semester. For
example, we will use this concept to explain why hurricanes can
intensify and
get as
strong as they do.
Try to reproduce this figure in your mind (together with the written
discussion and explanation) the next time you are lying in bed at night
trying to fall asleep. It'll put you right to sleep without any
of the side effects that medications might have.