Thursday Sept. 9, 2010
click here to download today's notes in a more
printer friendly format
3 songs ("White Winter Hymnal", "Tiger Mountain Peasant Song", and
"Mykonos") from the Fleet
Foxes before class today.
The 1st Optional (Extra Credit)
Assignment of the semester is now
available.
The 1st 1S1P Report Assignment is also
online.
If today's quiz were a real quiz you'd have the entire period to
work on it. But since this was just a Practice Quiz we did cover
a little bit of new material today.
This first section tries to explain how a mercury barometer
works. A mercury
barometer is used to measure atmospheric pressure and is really
just a balance that can be used to weigh the
atmosphere. You'll find a somewhat messier version of of what
follows on p. 29 in the
photocopied Class Notes.
 |
 |
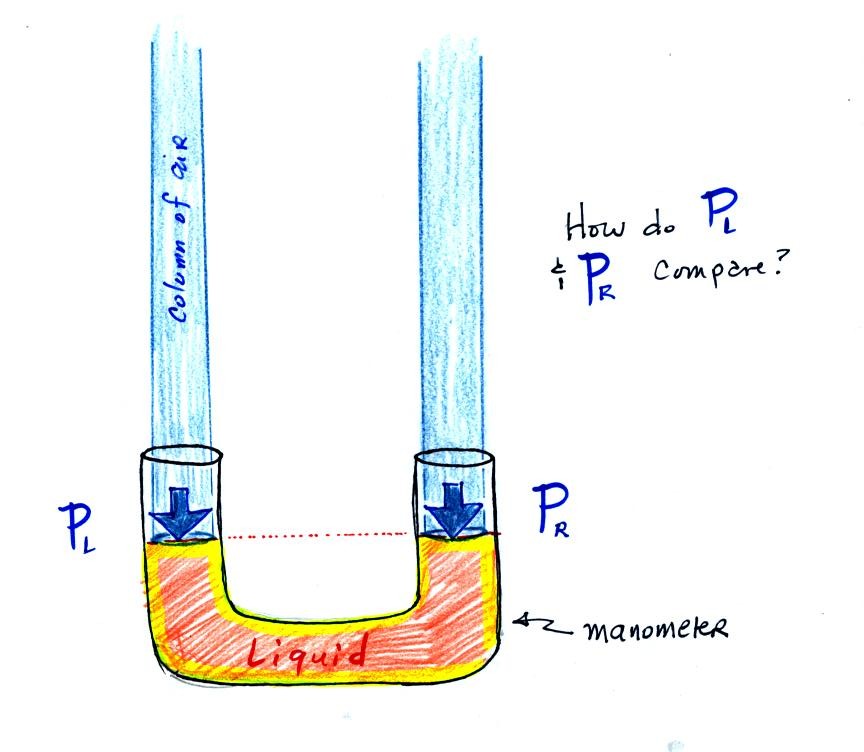
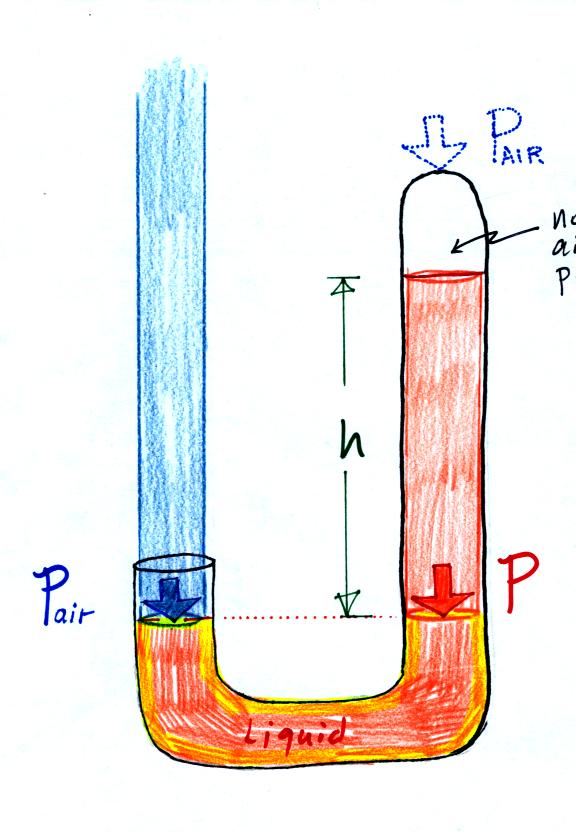
The instrument in the left figure
above ( a u-shaped
glass
tube filled with a
liquid of some kind) is actually called a manometer and can be used to
measure pressure
difference. The
two ends of the tube are open so that air can get inside and air
pressure can press on the liquid. Given that the liquid levels on
the two sides of the manometer
are equal, what could you about PL and PR?
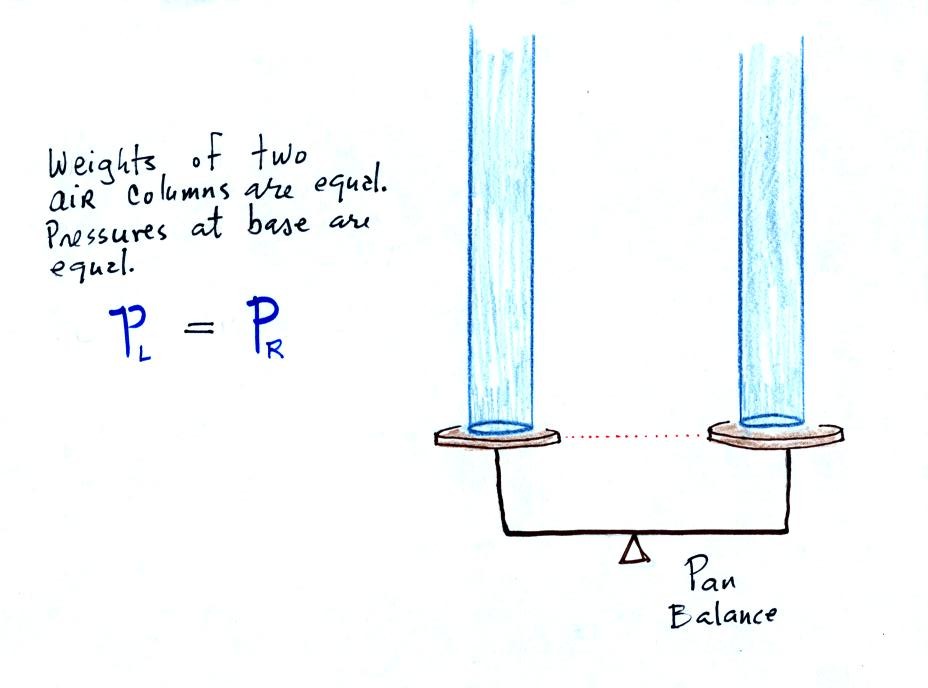
The liquid can slosh back and
forth just like the pans on a balance can move up and down. A
manometer really behaves just like a pan balance (pictured at
right) or a teeter totter (seesaw).
Because
the
two pans are in balance, the two columns of
air have the same weight. PL and PR
are equal (but note
that you don't really know what either pressure is just that they are
equal).
 |
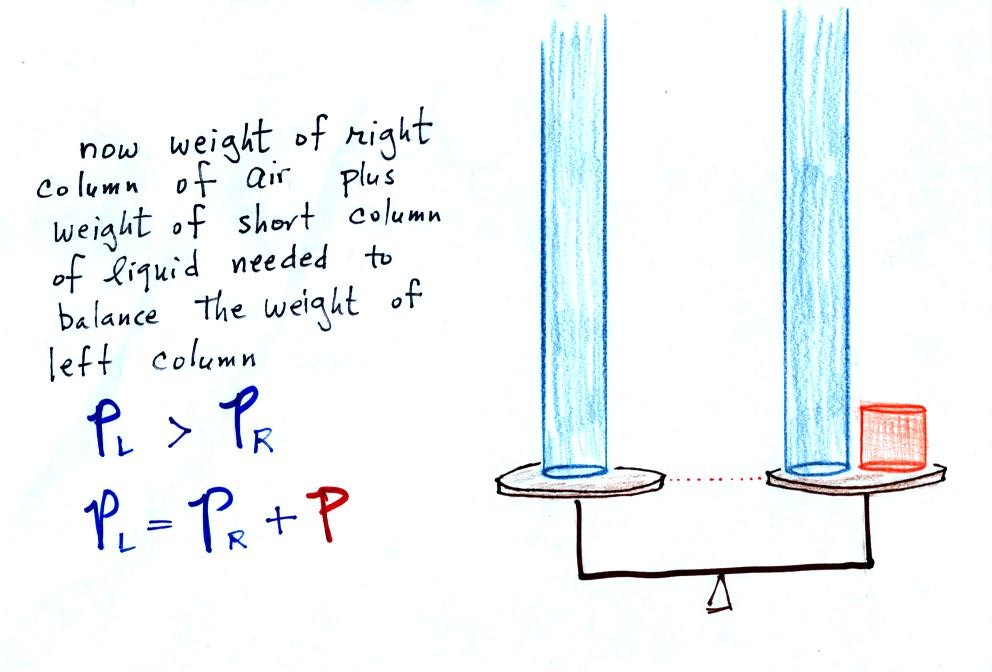 |
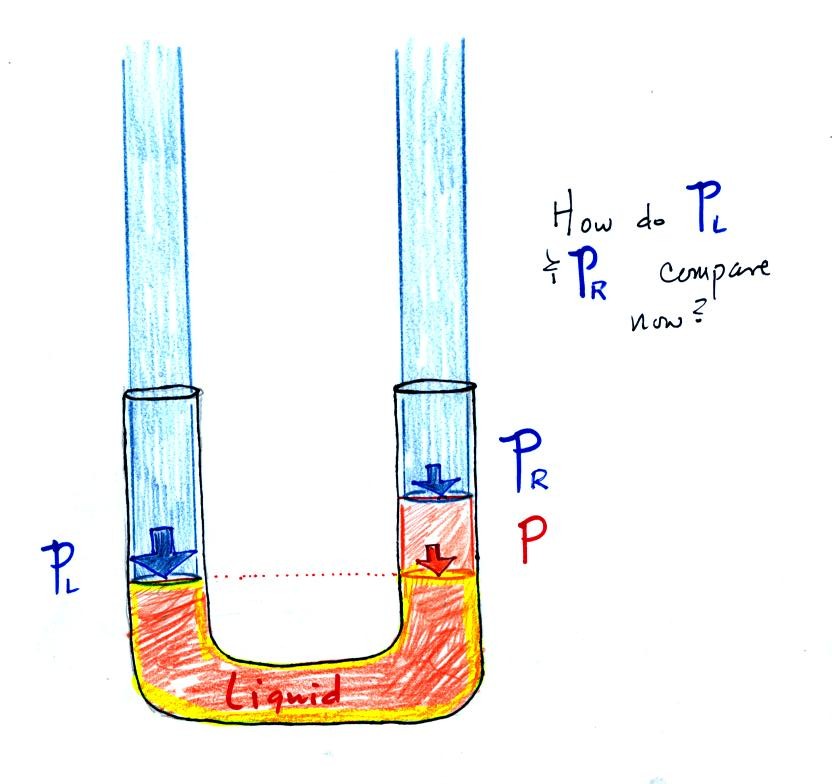
Now
the
situation is a little
different,
the
liquid levels
are no
longer equal. You probably realize that the air pressure on the
left, PL, is a little higher than the air pressure on the
right,
PR. PL is now being balanced by PR
+ P acting together. P
is the pressure produced by the weight of the extra fluid on the right
hand side of
the manometer (the fluid that lies above the dotted line). The
height
of
the
column
of
extra
liquid
provides
a measure of the difference between PL and PR.
Next we will just go and close off
the right hand side of the
manometer.
|
|
Air pressure can't get into the
right tube any
more. Now at the level of the dotted line the balance is between
Pair and P (pressure by the extra liquid on the
right). If
Pair
changes, the height of the right column, h, will
change. You now have a barometer, an instrument that can measure
and monitor the atmospheric pressure. (some of the writing
in the upper right portion of the left figure was cut off, it should
read "no air
pressure")
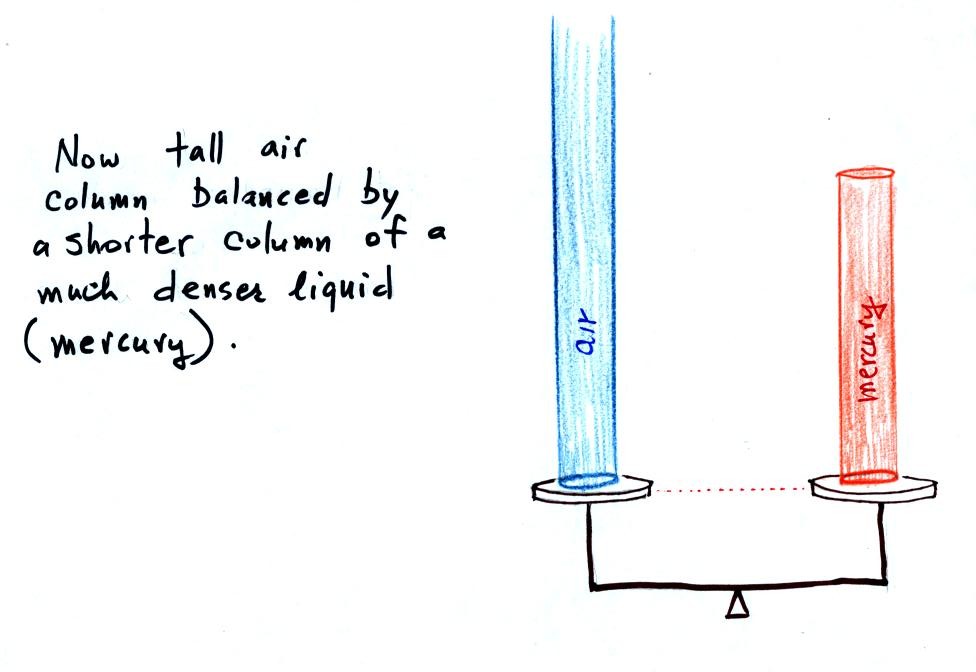
Barometers like this are usually
filled with mercury. Mercury is
a liquid. You need a liquid that can slosh back and forth in
response to changes in air pressure. Mercury is also very dense
which
means the barometer won't need to be as tall as if you used something
like water. A water barometer would need to be over 30 feet
tall. With mercury you will need only a 30 inch tall column to
balance the weight of the atmosphere at sea level under normal
conditions (remember the 30 inches of mercury pressure units mentioned
earlier). Mercury also has a low rate of
evaporation so you don't have much mercury gas at the top of the right
tube (it is the mercury vapor that would make a mercury spill in the
classroom dangerous).

Here is a more conventional
barometer design.
The bowl of
mercury is usually covered in such a way that it can sense changes in
pressure but is sealed to keep poisonous mercury
vapor from filling a room.
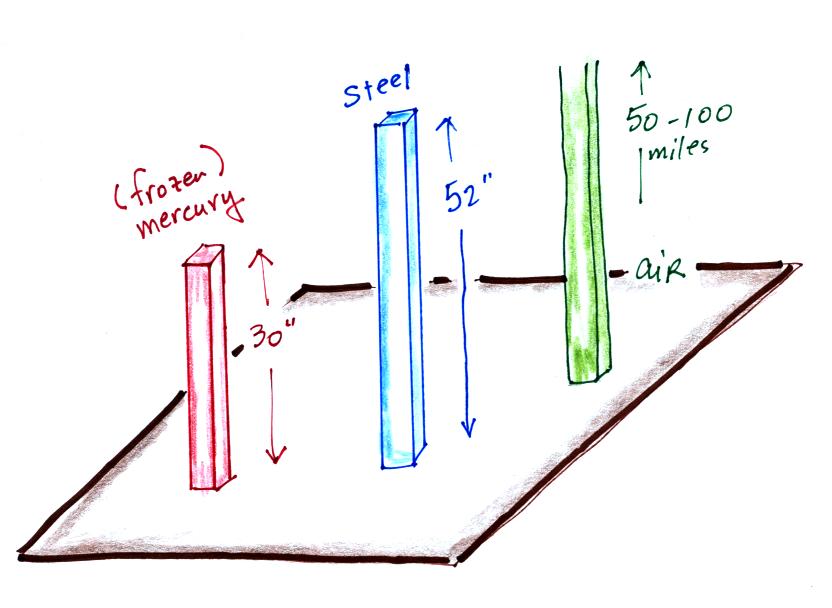
In an earlier class we saw that a 52 inch long 1"x1" steel bar
weighs the same as a 1" x 1" column of air stretching from sea level to
the top of the atmosphere. Now we can add a 30 inch tall 1" x 1"
column of mercury (frozen so that it would be rigid) to the list.
All three columns above would weigh 14.7 pounds. They would all
be pushing against the ground with a pressure of 14.7 psi.
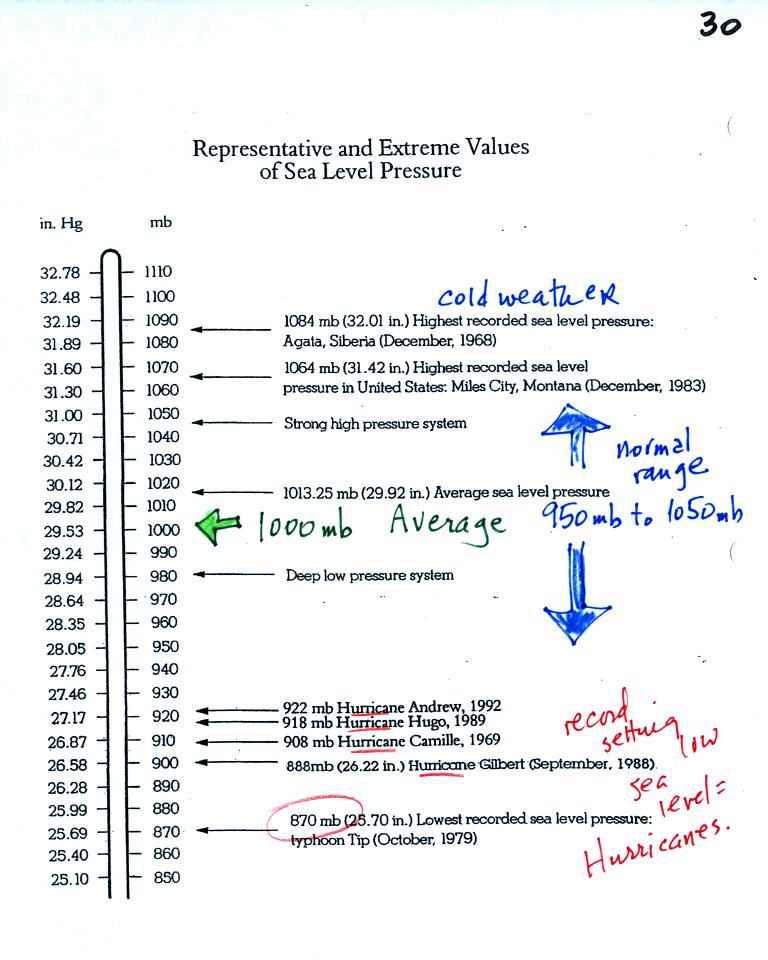
The figure above (p. 30 in the
photocopied Class Notes)
first
shows average sea level pressure values. 1000 mb or 30 inches of
mercury are close enough in this class.
Sea level pressures
usually fall between 950 mb and 1050 mb.
Record high sea level
pressure values occur during cold weather. The TV weather
forecast will often associated hot weather with high pressure.
They are generally referring to upper level high pressure (high
pressure at some level above the ground) rather than surface pressure.
Record low pressure
values have all been set by intense hurricanes (the record setting low
pressure is the reason these storms were so intense). Hurricane
Wilma in 2005 set a new record low sea level pressure reading for the
Atlantic. Hurricane Katrina had a pressure of 902 mb.
The following table lists some of the information on hurricane strength
from p. 146a in the photocopied ClassNotes. 3 of the 10 strongest
N. Atlantic hurricanes occurred in 2005.
Most
Intense
North
Atlantic
Hurricanes
|
Most
Intense
Hurricanes
to
hit
the
US
Mainland
|
Wilma
(2005)
882
mb
Gilbert (1988) 888 mb
1935 Labor Day 892 mb
Rita (2005) 895 mb
Allen (1980) 899
Katrina (2005) 902
|
1935
Labor
Day
892 mb
Camille (1969) 909 mb
Katrina (2005) 920 mb
Andrew (1992) 922 mb
1886 Indianola (Texas) 925 mb |
Pressure
at
any
level in the
atmosphere depends on (is determined by) the weight of the air
overhead. We used a pile of bricks (each brick represents a layer
of air)
to help visualize and understand why pressure decreases with
increasing altitude. A pile of bricks and columns of air or
mercury on a pan balance can lead to the believe
that
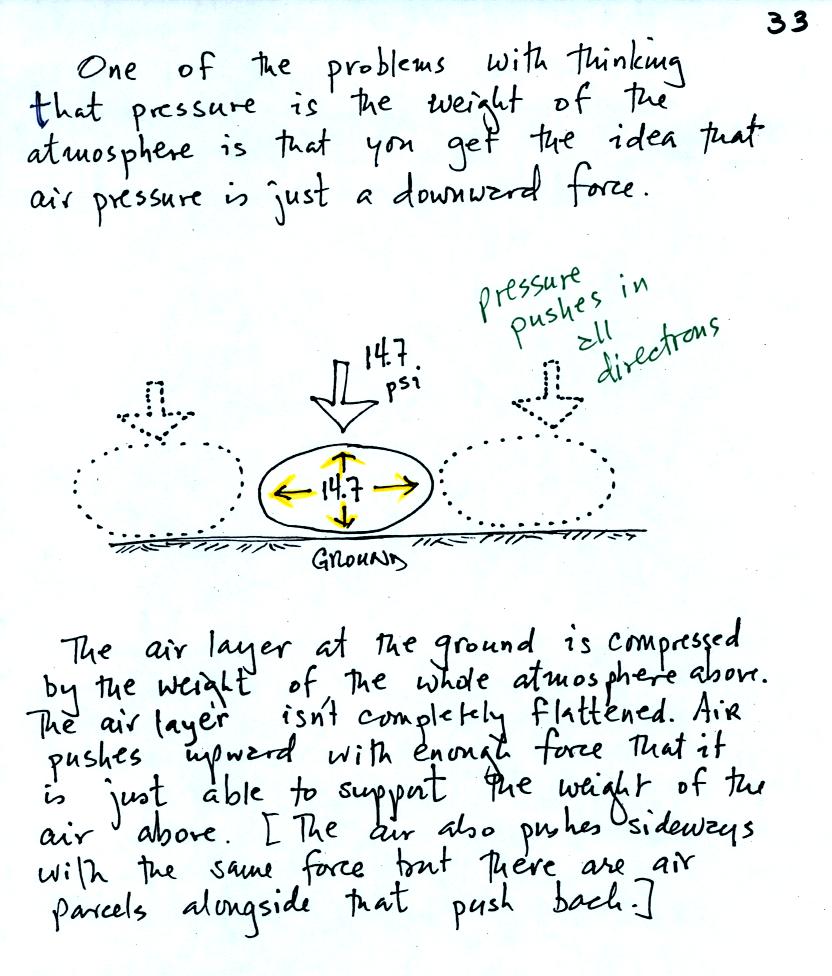
air pressure exerts force in just a downward direction.
Air pressure is a force that pushes
downward, upward, and
sideways.
If you fill a balloon with air and then push downward on it, you can
feel the air in the balloon pushing back (pushing upward). You'd
see the air in the balloon pushing sideways as well.
The air
pressure in the four tires on your automobile pushes
pushes upward
with enough force to keep the 1000 or 2000 pound vehicle off the
road.
A better
representation of air in the atmosphere might be a people pyramid.
If the bottom person in the stack above were standing on a
scale, the
scale would measure the total weight of all the people in the
pile. That's analogous to sea level pressure being determined by
the weight of the all the air above.
The bottom person in the
picture above must be strong enough to support the weight of all the
people above. That is equivalent to the bottom layer of the
atmosphere having enough pressure, pressure that points up, down, and
sideways, to support the weight of the air above.
This was a logical point to do a demonstration. A demo that
tries to prove that air pressure really does push upward as well as
downward. Not only that but the upward force is fairly
strong. The demonstration is summarized on p. 35 a in the
ClassNotes.
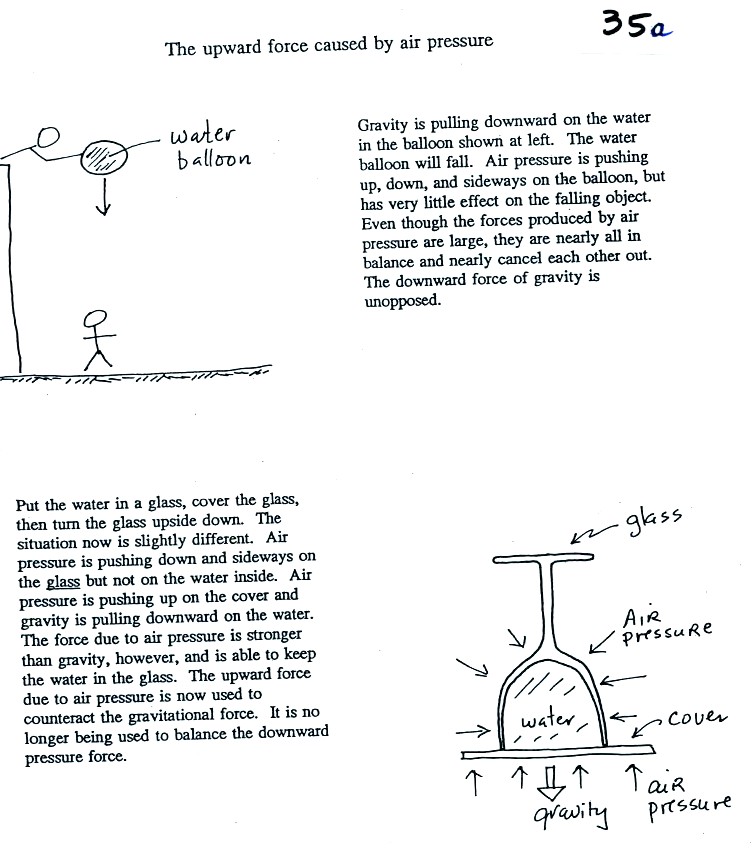
Here's a little bit more detailed and more complete explanation of
what is going on. First the case of a water balloon.
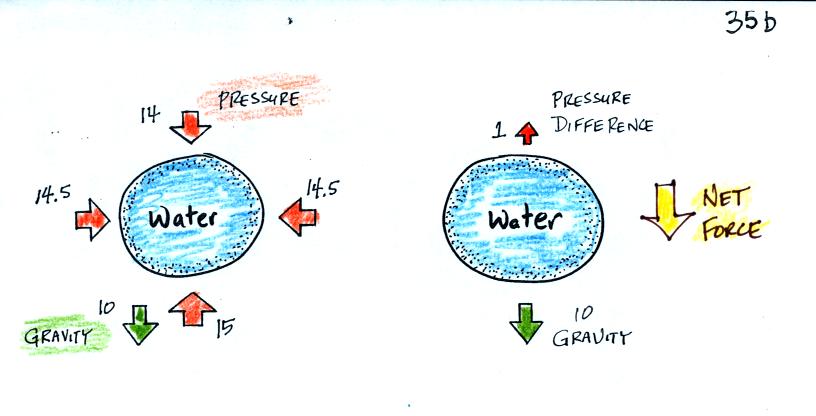
In the demonstration a wine glass is filled with water. A small
plastic lid is used to cover the wine glass. You can then turn
the glass upside down without the water falling out.
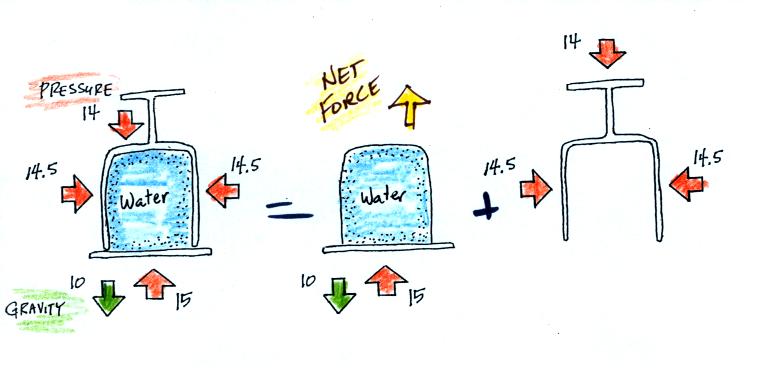
All the same forces are shown again in the left most
figure.
In
the right two figures we separate this into two parts. First
the water inside the glass isn't feeling the downward and sideways
pressure forces (because they're pushing on the glass, this is shown at
the right side of the figure above). Gravity
still pulls downward on the water but the upward pressure force is able
to overcome the downward pull of gravity. The upward pointing
pressure force is used to overcome gravity not to cancel out the
downward pointing pressure force.
The demonstration was repeated using a 4 Liter flash (more than a
gallon of water, more than 8 pounds of water). The upward
pressure force was still able to keep the water in the flask (much of
the weight of the water is pushing against the sides of the flask which
the instructor was supporting with his arms).