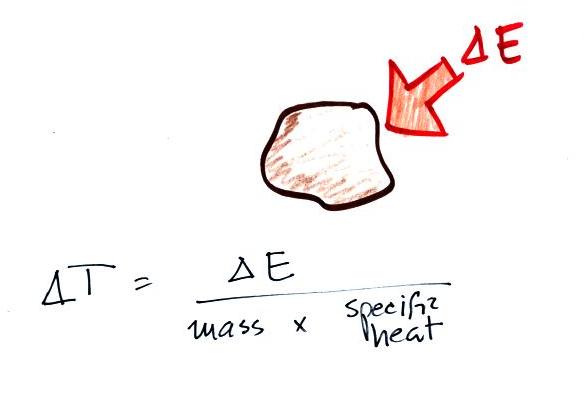
Here's the equation that allows you
to determine how much of a temperature change will occur when energy is
added to or removed from an object. What exactly is happening
inside an object when it's temperature changes?
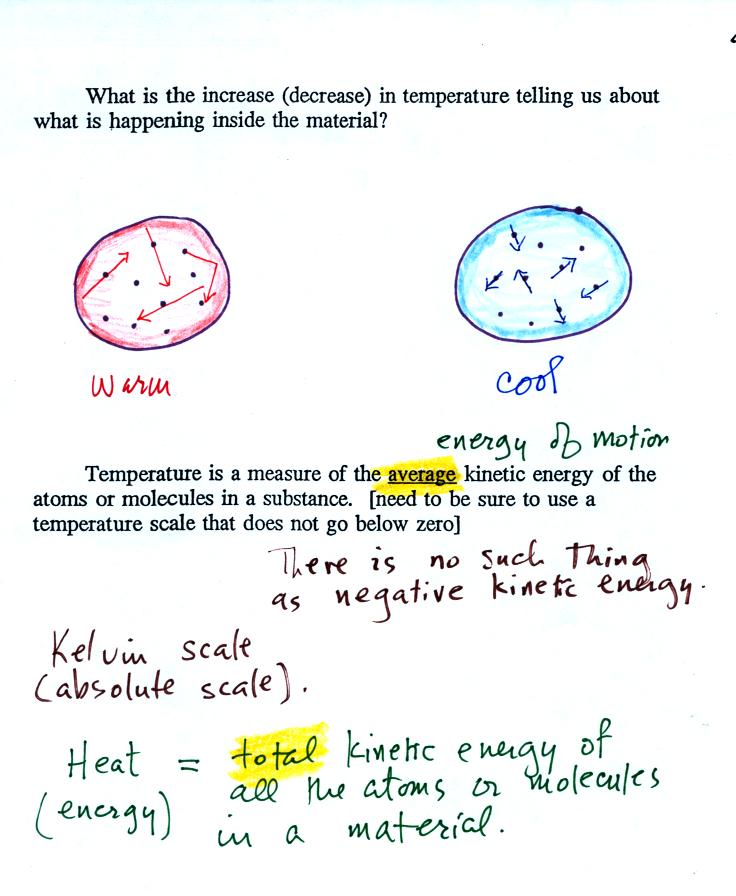
The figure above is on p. 46 in the
photocopied Class
Notes. Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material. The atoms or molecules in a cold
material will be moving more slowly than the atoms or molecules in a
warmer object.
You need to be careful what temperature scale you use
when
using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero (0 K is known as absolute zero). The smallest kinetic
energy you can have is zero
kinetic energy. There is no such thing as negative kinetic energy.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
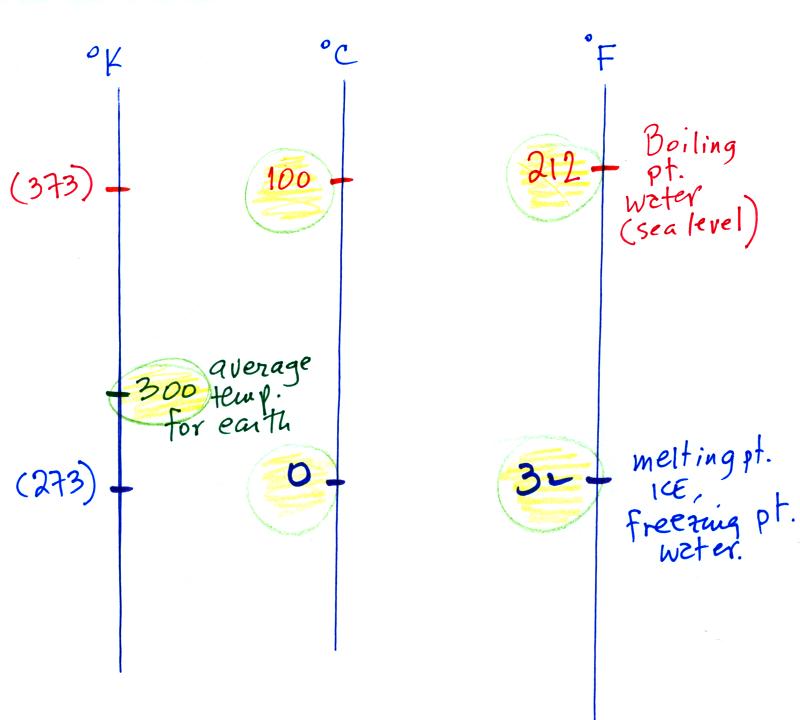
Speaking of temperature scales
You should remember the
temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and perhaps the Kelvin
scales. 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth.
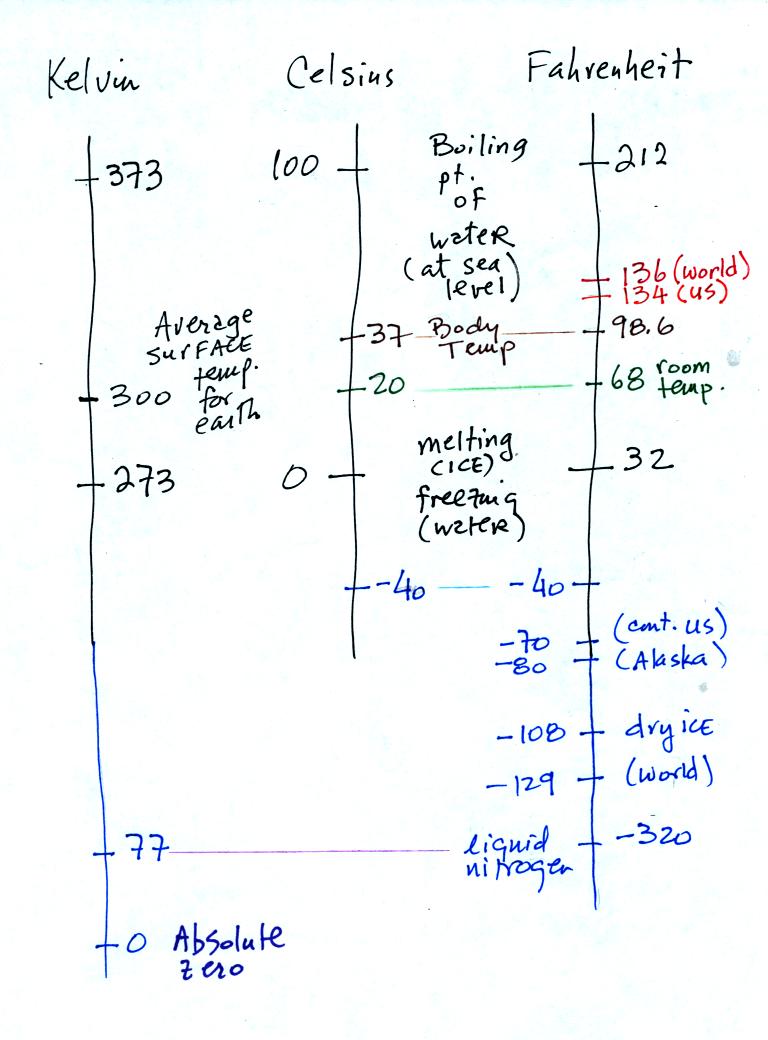
You certainly don't need to try to
remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than being
near or
surrounded by ocean. Liquid
nitrogen is cold but it is still quite a bit warmer than absolute zero.
This next figure might make clearer the difference between
temperature (average kinetic energy) and heat (total kinetic energy).

A cup of water and a pool of water
both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So if you add together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people shown have the same
average
amount
of money per person (that's analogous to temperature). The $100
held by the larger group at the
left is
greater than the $20 total possessed by the smaller group of people on
the right (total amount of money is analogous to heat).
It was time for a little bit of a
break from the normal routine in NATS 101 at this point.
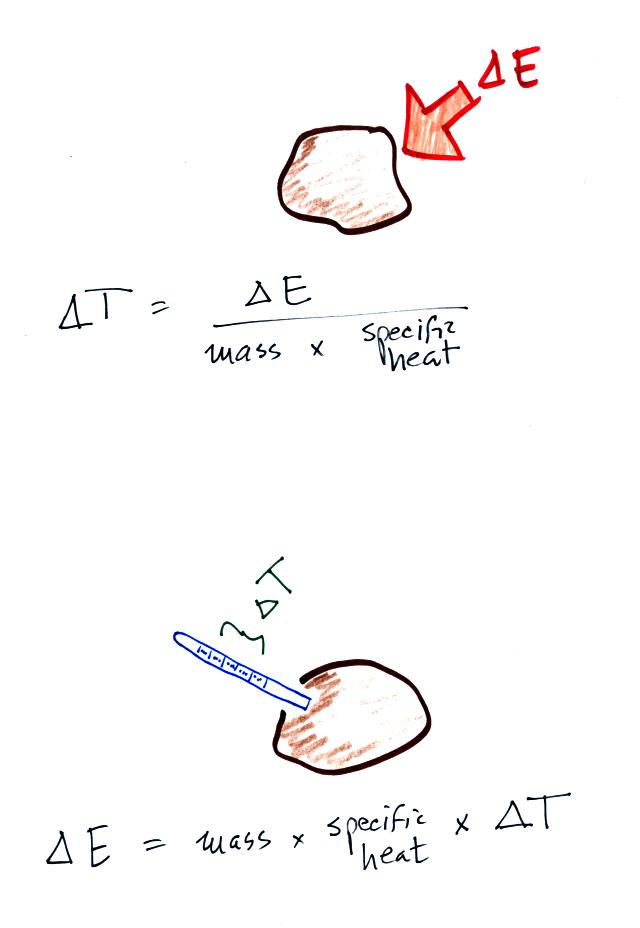
If you add energy to or remove
energy from an object, the
object
will usually change temperature. You can calculate the
temperature change if you know the object's mass and its specific
heat. That's the equation we used in the example calculation
earlier.
You can rearrange the terms in the top equation to get the bottom
equation in the figure above. In this case you can measure the
temperature change that an object experiences and use that to determine
the amount of energy that was added to or removed from the
object. We used this rearranged equation in a class experiment.
Three students from the class were courageous enough to volunteer
to
perform the experiment (actually the students were told they could to
use the
data they collect to write their experiment report which satisfies part
of the
writing requirment for this class).
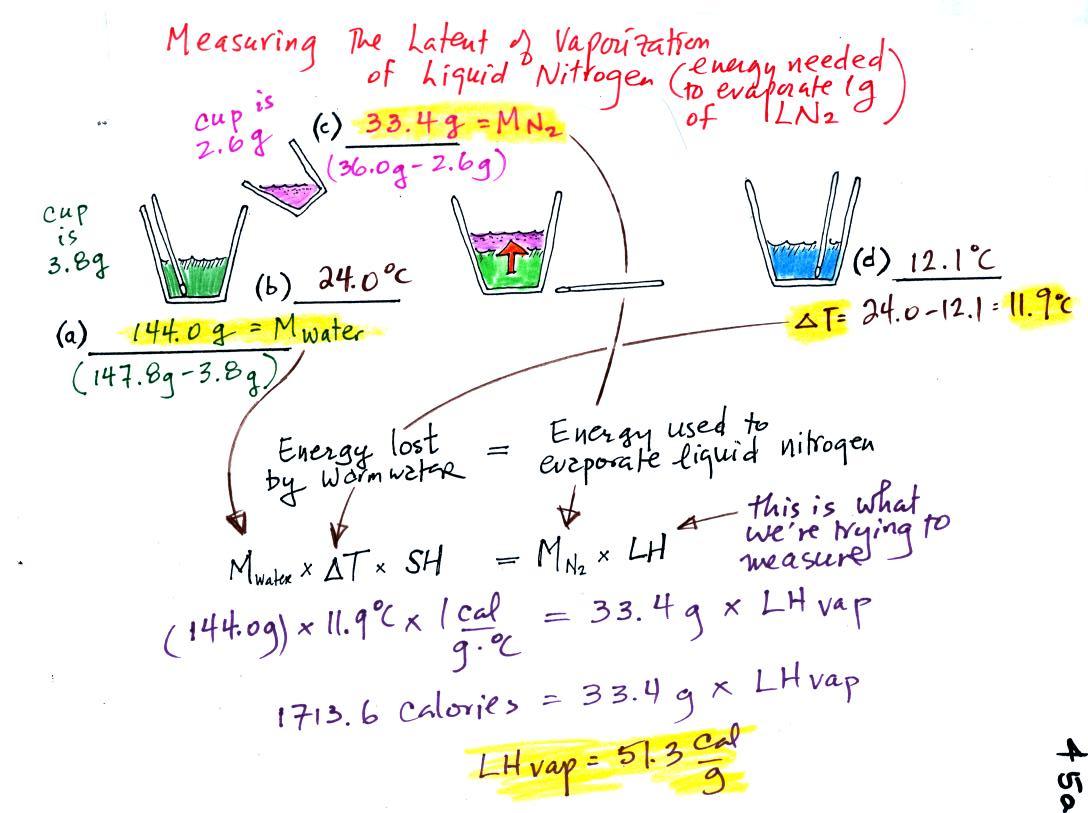
The object of the experiment was to
measure the latent heat of
vaporization of liquid nitrogen. That just means measuring the
amount of energy needed to evaporate a gram of liquid nitrogen (this is
very similar to Experiment #2 where students are measuring the latent
heat
of fusion of ice, the energy needed to melt a gram of ice).
You'll
find the following figure on p. 45a in the photocopied
Classnotes. The students will each pour a known amount
(known mass) of liquid nitrogen into a cup of water. The students
will measure the temperature change of a
small cup of water and
use that to
determine the amount of energy lost by the water.
(a)
Some room temperature water poured into a styrofoam cup weighed
147.8
g. The cup itself weighed 3.8 g, so the cup contained 144.0 g of
water.
(b)
The water's temperature was 24.0 C (room temperature).
(c)
33.4 g of liquid nitrogen was poured into the cup of water.
It takes energy to turn liquid nitrogen into nitrogen gas.
The needed energy came from the water. This flow of energy is
shown in the middle figure above. We assumed that because the
experiment is performed in a styrofoam cup that there is no energy
flowing between the water in the cup and the surounding air. All
of the energy leaving the water is being used to evaporate nitrogen
(d)
After the liquid nitrogen had evaporated we remeasured the water's
temperature. It had dropped to 12.1 C. That is a
temperature drop of 23.0 - 12.1 = 11.9 C. Note:
these final temperature and temperature drop values are different from
the ones collected and written down in class. You may remember
that the calculated latent heat of vaporization value in class wasn't
very close to the known value. After looking at all three
students data after class we determined that the final temperature
reading in class was wrong and we repeated the measurement. The
numbers from the second experiment are being used above.
Because we knew how
much water we started with, its temperature drop, and water's specific
heat we can calculate how much
energy was taken from the water.
144.0 g. x 11.9 C x 1 cal/(g C) = 1713.6 calories
We then divide that number by the amount of liquid nitrogen that
was
evaporated.
1713.6 calories / 33.4 g grams = 51.3
calories per gram
So that's what we measured.
How close did we get to the known value? At the beginning of the
experiment an envelop had been given to a trustworthy student in the
class (though unfortunately not a Buddhist Monk).

The known value was inside

Our measured value was very close
to the known value of 48 cal/g. The two other students measured
43.8 cal/g and 50.0 cal/g. The average of all three measurements
is 48.4 cal/g.
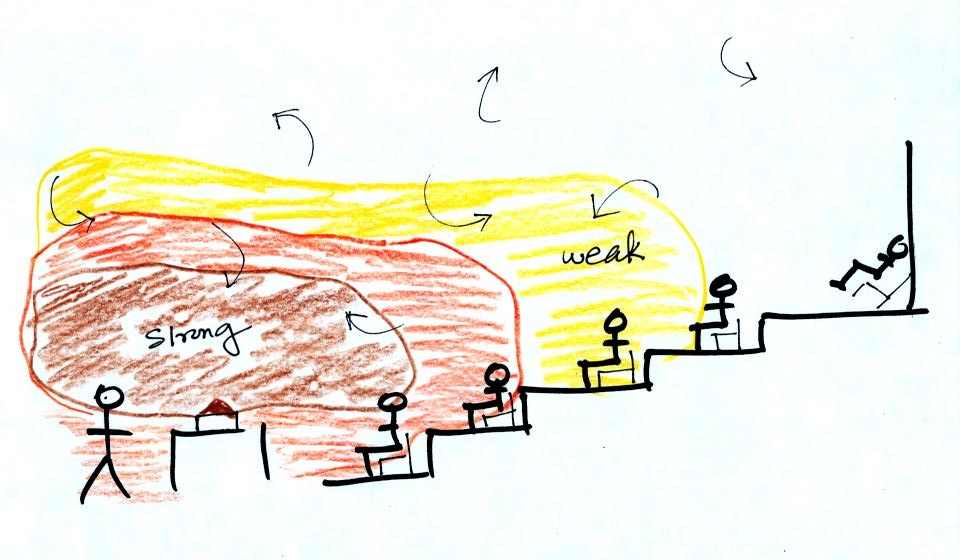
Conduction
is the first of four energy transport processes
that we
will cover (the least important transport process in the
atmosphere). The figure below illustrates this process. A
hot object is stuck in the middle of some air.
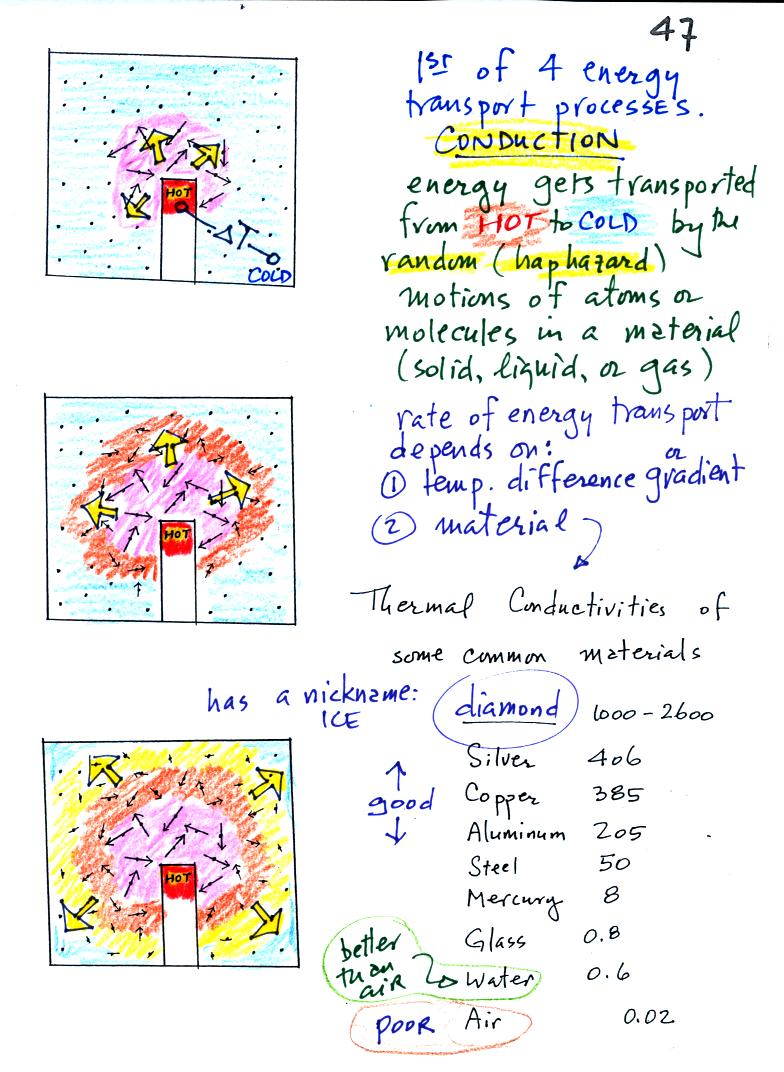
In the top picture some of the
atoms or molecules near the
hot object have collided with the object and picked up energy from the
object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored pink).
In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object.
In
the third picture molecules further out have now (the yellow ones)
gained
some energy. The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder material.
Conduction transports energy from hot to cold. The rate of
energy transport depends first on the material (air in the example
above). Thermal
conductivities of some common materials are listed. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors (cooking pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on
temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
Transport of energy by conduction is similar to the
transport of a strong smell throughout a classroom by diffusion.
Small eddies of wind in the classroom blow in random directions and
move smells throughout the room.. For our demonstration we used
curry powder.
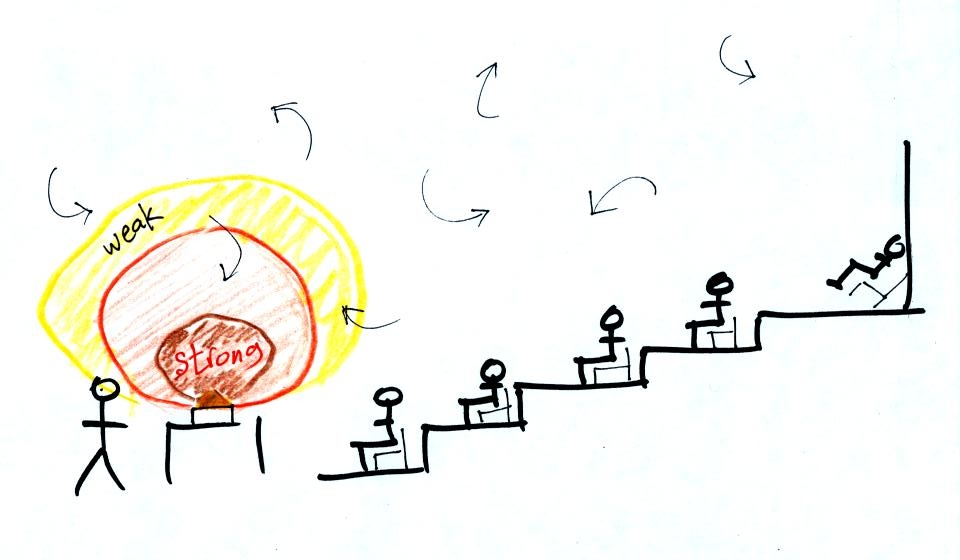
Because
air has such a low thermal conductivity it is often used as an
insulator. It is important, however, to keep the air trapped in
small pockets or small volumes so that it isn't able to move and
transport energy by convection (we'll look at convection
shortly). Here are some examples of
insulators that use air:
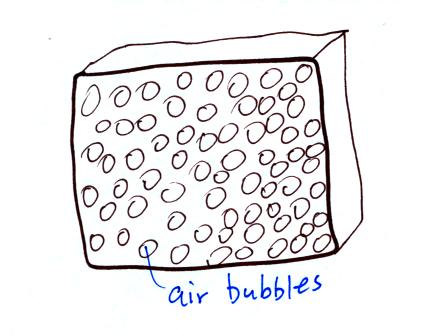
Foam is
filled with lots of small air bubbles
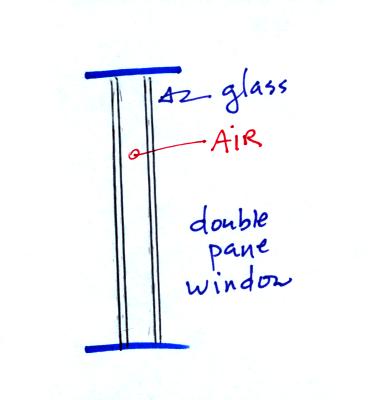
Thin
insulating layer of air in a double
pane window
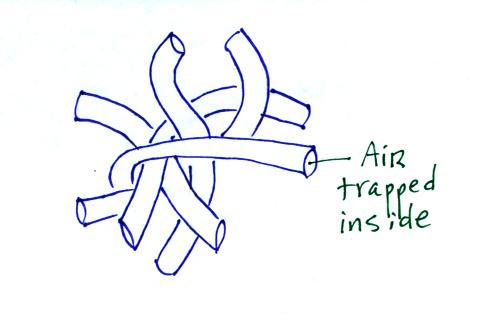
Hollow fibers
(Hollofil) filled with air used in
sleeping
bags and
winter coats. Goose feathers
(goosedown) work in a similar way.
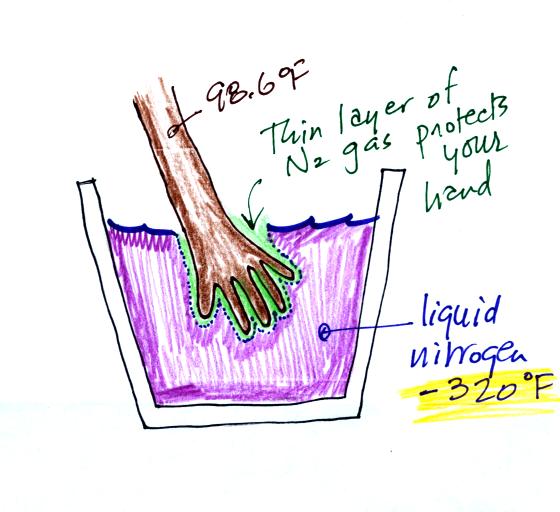
You can safely stick your
hand in liquid nitrogen for a fraction of a second. It doesn't
feel particularly cold and doesn't feel wet. Some of the liquid
nitrogen quickly evaporates and surrounds your hand with a layer of
nitrogen
gas. This gas is a poor conductor and insulates your
hand from the cold for a short time (the gas is a poor conductor but a
conductor nonetheless; if you leave your hand in the liquid nitrogen
for very long it will freeze and your hand would need to be amputated).
Convection
was the next energy transport process we had a look at. Rather
than moving about randomly, the atoms or molecules move as a
group (organized motion). Convection works in liquids and gases
but not
solids (the atoms or molecules in a solid can't move freely).
At Point 1 in the picture above a
thin layer of air
surrounding a hot object has
been
heated by conduction. Then at Point 2 a person (yes, that is a drawing
of a
person's head) is blowing the blob of warm air
off to the right. The warm air molecules are moving away at Point
3 from the
hot object together as a group (that's the organized part of the
motion). At Point 4 cooler air moves in and surrounds the hot
object and the whole process can repeat itself.
This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly. I put a
small fan behind the curry powder to help spread the
smell faster and further out into the classroom (it nearly blew some of
the powder into the eyes of students sitting in the front row in the
MWF section of the class).
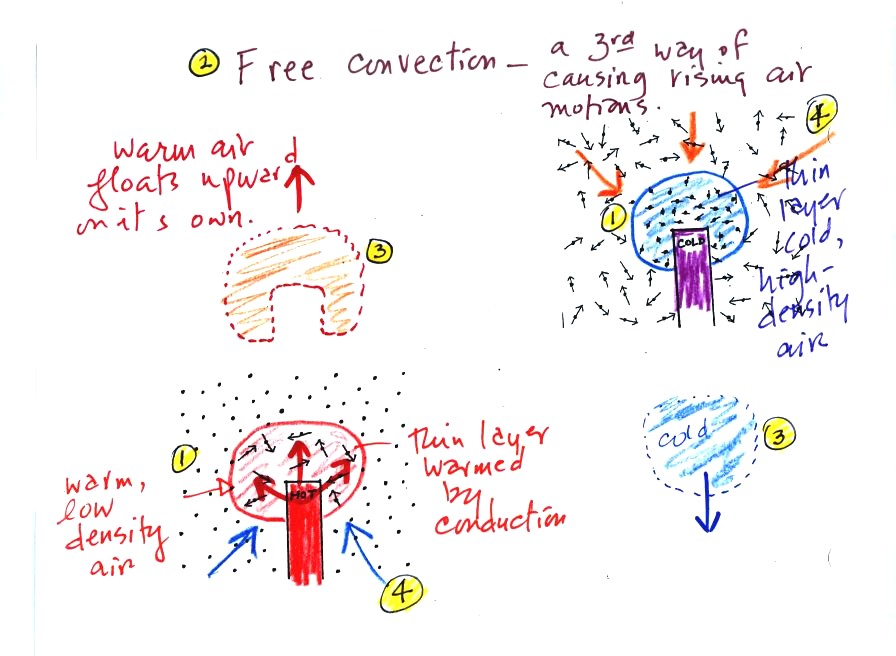
A thin layer of air at Point 1 in
the figure above (lower
left) is
heated by conduction. Then because hot air is also
low density air, it actually isn't necessary to blow on the hot object,
the
warm air will rise by itself (Point 3). Energy is being
transported away
from the hot object into the cooler surrounding air. This is
called free convection. Cooler air moves in to take the place of
the rising air at Point 4 and the cycle repeats itself.
The example at upper right is also
free convection. Room temperature air in contact with a cold
object loses energy and becomes cold high density air. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the room temperature surroundings to the
colder object.
In both examples of free convection, energy is being transported
from
hot toward cold.
Now some
what I think are some fairly practical applications of what we have
learned about conductive and
convective energy transport. Energy transport really does show up
in a lot more everyday real life situations than you might expect.
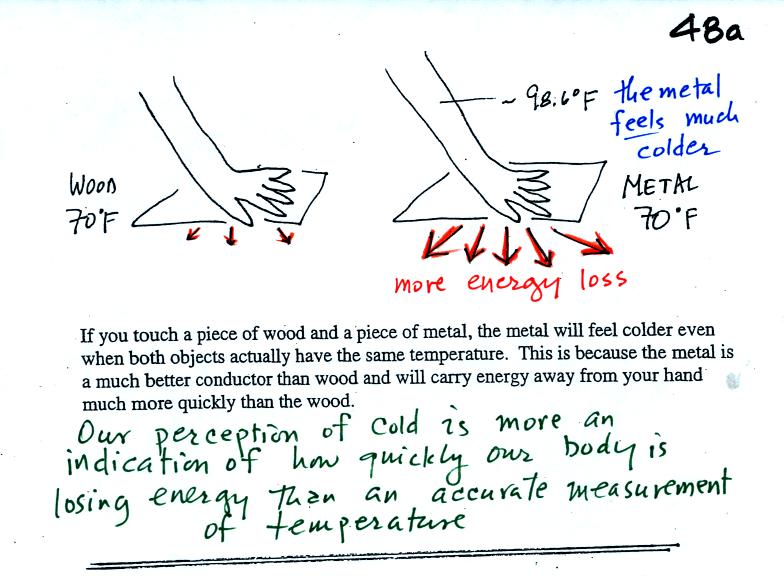
Note first of all there is a temperature difference between
your hand and a 70 F object. Energy will flow from your warm
hand to the colder object. Metals are better conductors than
wood. If you touch a
piece of
70 F metal it will feel much colder than a piece of 70 F wood, even
though they both have the same temperature. A
piece
of 70 F diamond would feel even colder because it is an even better
conductor
than metal. Pieces of metal and wood were passed around class so
that you could feel the difference (though the piece of metal had
warmed noticeably by the time it had been returned)
Something that feels cold may not be as
cold as it seems. Our perception of cold is more an
indication of how
quickly our hand is losing energy than a reliable measurement of
temperature.
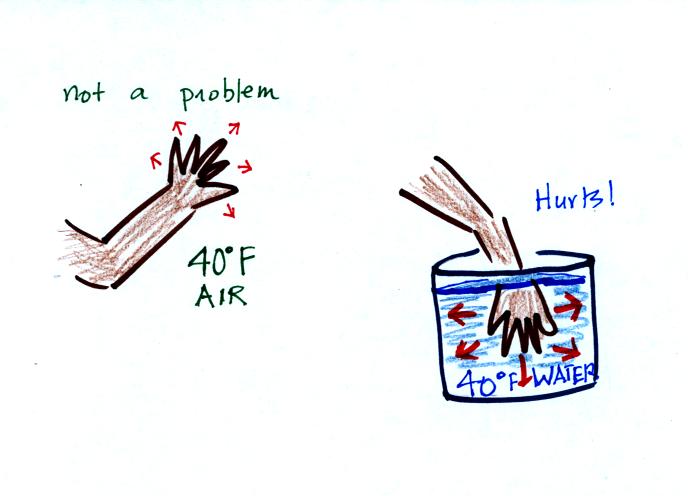
Here's a similar situation.
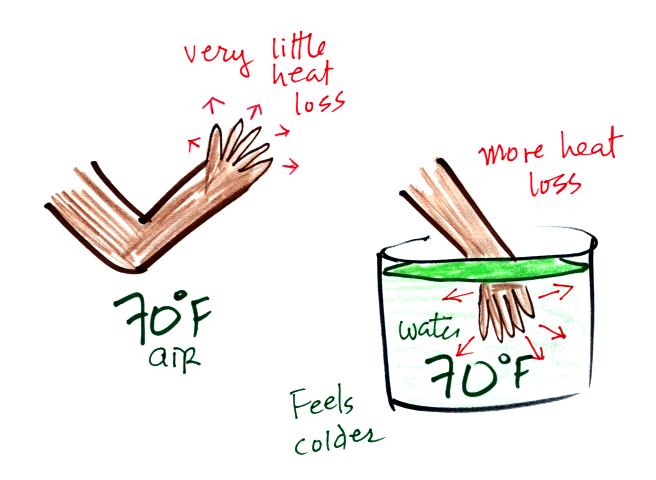
It's pleasant
standing outside on a nice day in 70 F air. But if
you jump into 70 F pool water you
will
feel cold, at least until you "get used" to the water temperature (your
body might reduce blood flow to your extremeties and skin to try to
reduce energy loss).
Air is a poor conductor. If you go out in
40 F
weather you will feel colder largely because there is a larger
temperature difference between you and your surroundings (and
temperature difference is one of the factors that affect rate of energy
transport by conduction).
If you stick your hand
into a bucket of 40 F water (I probably shouldn't suggest you try this,
but...), it will feel very cold (your hand will
actually soon begin to hurt). Water is a much better conductor
than air. Energy flows much more rapidly from your hand into the
cold water.
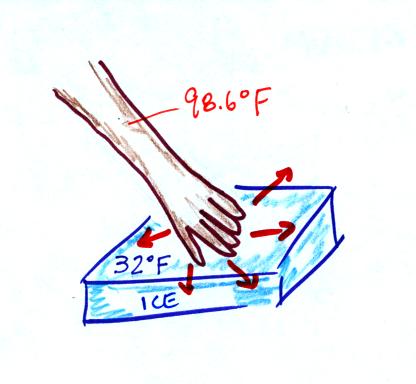
Ice
feels cold even though it is not a particularly good
conductor. This is because of the large temperature difference
between your hand and the water. This figure wasn't
shown in class.
Our
perception of cold is a better indicator of how quickly our body is
losing energy rather than an accurate measurement of temperature.
This basic knowledge puts us in a perfect position to understand the
concept of wind
chill temperature.
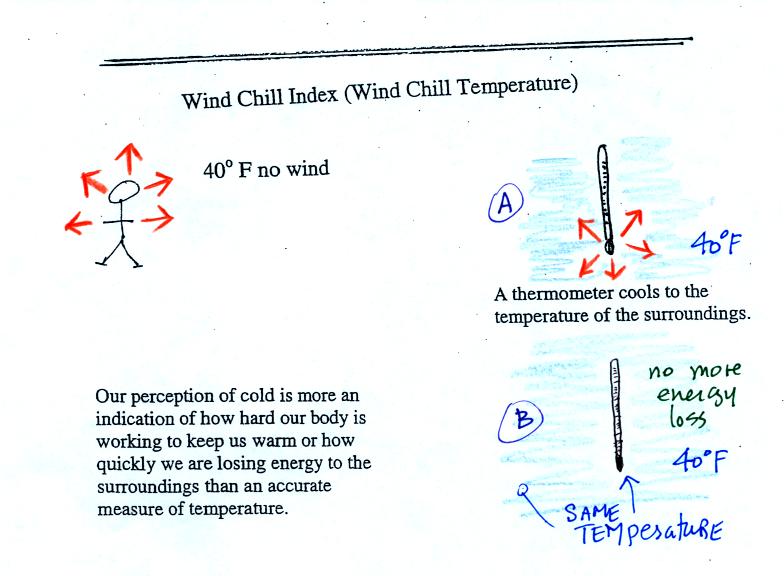
Your body works hard to keep its core temperature around
98.6 F. If you go outside on a 40 F day (calm winds)
you will
feel
cool; your
body is losing energy to the colder surroundings (by conduction
mainly). Your body will be able to keep you warm for a little
while anyway (maybe indefinitely, I don't know). A thermometer
behaves differently, it is supposed to cool to the temperature of the
surroundings. Once it reaches 40 F it won't lose any additional
energy. If your body cools to 40 F you will probably die.
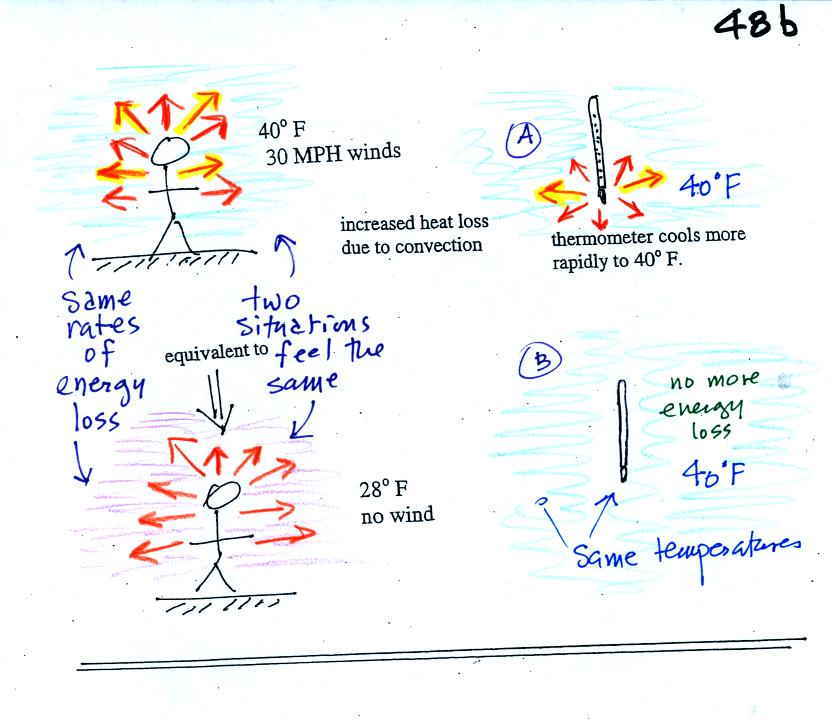
If you go outside on a 40 F day with 30 MPH winds your
body
will lose
energy at a more rapid rate (because convection together with
conduction are transporting energy away from your body). Note the
additional arrows drawn on the figures above indicating the greater
heat loss. This
higher rate of energy loss will make it feel colder
than a 40
F day
with calm winds.
Actually, in terms of the rate at which your
body loses energy, the windy 40 F day would feel the same as a 28
F day without any wind. Your body is losing energy at the same
rate in both
cases. The combination 40 F and 30 MPH winds results in a wind
chill temperature of 28 F.
The thermometer will again cool to the
temperature of its surroundings, it will just cool more quickly on a
windy day. Once the thermometer reaches 40 F there won't be any
additional energy flow. The
thermometer would measure 40 F on both the calm and the windy day.
Standing outside on a 40 F day is not an immediate life
threatening
situation. Falling into 40 F water is.
Energy will be conducted away from your body more quickly
than
your
body can replace it. Your core body temperature will drop and
bring on hypothermia.
Be
sure
not
to
confuse
hypothermia
with
hyperthermia
which
can bring on
heatstroke and also a serious outdoors risk in S.
Arizona.