Friday August 26, 2011
click here
to download a copy of today's notes in a more printer friendly format
Iron and Wine provided the background music while Experiment #1 materials were distributed
before class today ("The Devil Never
Sleeps", "Jezebel"
and
"Freedom Hangs
Like Heaven").
Coming next Monday TA office hours and (hopefully) the first optional
homework assignment of the semester.
Some information about haboobs (dust storms) and the thunderstorms
that cause them during the first portion of class today. This was
in response to a student question after class last Wednesday.
You'll find this material in the middle of the Wednesday
Aug.
24 lecture notes. The section includes two time lapse
videos of a pretty spectacular haboob that moved through the Pheonix
area earlier this summer (July 5).
Next we finished up the short section that we started on Wednesday
concerning the origin and evolution of the atmosphere. The
following figure is the first page in the packet of photocopied
ClassNotes.
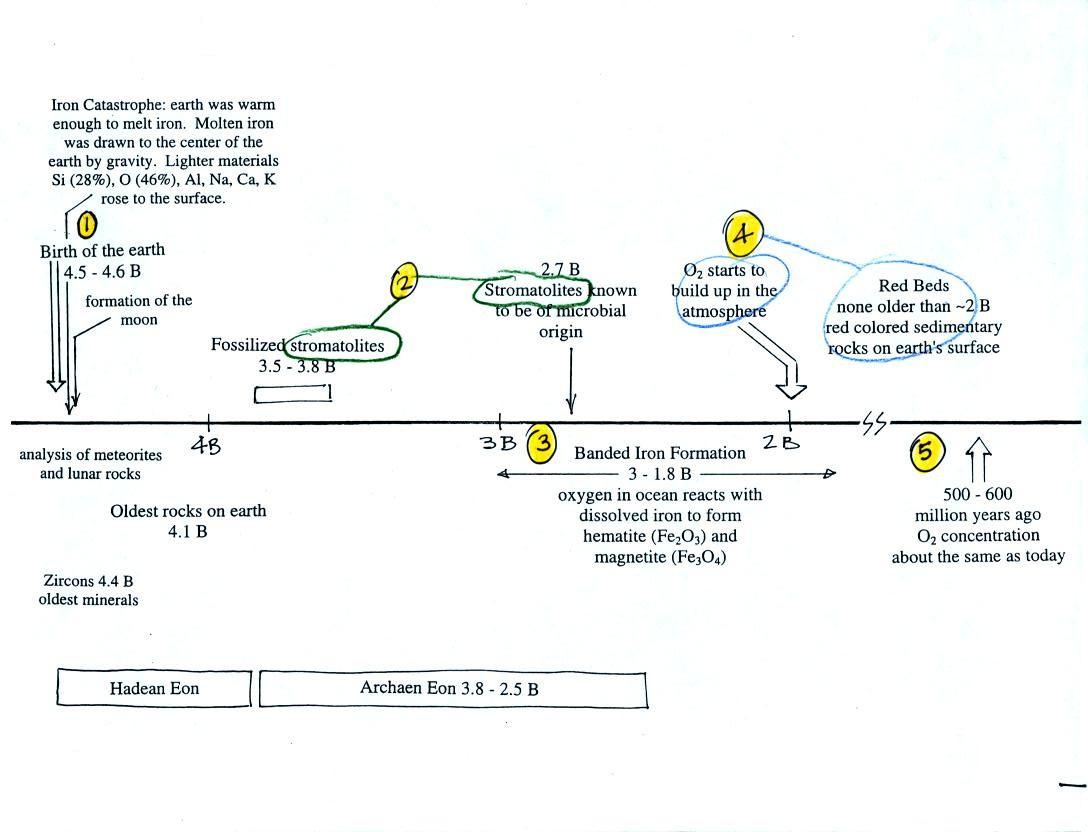
This somewhat confusing
figure shows some of the important events in the history of the earth
and evolution of the atmosphere. The numbered points were
emphasized.
First, Point 1: the earth
is thought to be between 4.5
and 4.6 billion years old. If you want to remember the earth is a
few
billion years old that is probably close enough.
Stromatolites
(Point
2)
are
column-shaped
structures
made
up of layers of sedimentary rock, that are created by
microorganisms
living at the top of the stromatolite (I've never actually seen a
stromatolite, so this is all based on photographs and written
descriptions). Fossils of the very small microbes (cyanobacteria
= blue green algae)
have been found in stromatolites as old as 2.7 B years and are some of
the earliest records of life on earth. Much older (3.5 to 3.8
B years old) stromatolites presumably also produced by microbes, but
without
microbe fossils, have been found.
We're learning about
stromatolites
because the cyanobacteria were able to produce oxygen using
photosynthesis.
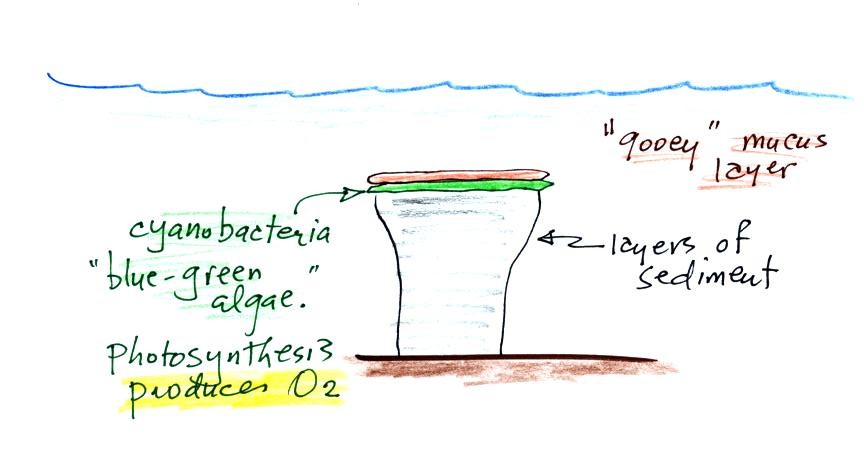
Living stromatolites are found
in a
few locations today. The picture above is from Coral Bay, located on
the
western tip of Australia. The picture was probably taken at
low tide, the stromatolites would normally be covered with ocean
water. It doesn't look like a good place to go swimming, I would
expect the top surfaces of these stromatolites to be slimy.
Here are a couple of pictures of
the samples of banded iron formation rock that was passed around in
class.
The main thing to notice are the alternating bands of red and black
rock. The next paragraph and figure explain how these formed.
Rain would first of all wash iron ions from the earth's land surface
into the ocean (at a time before there was any oxygen in the
atmosphere). Oxygen from the cyanobacteria living in the ocean
water reacted with the dissolved iron (the iron
ions) to form hematite or magnetite. These
two minerals precipitated out of the water to form a layer on the sea
bed. This produced the black layers.
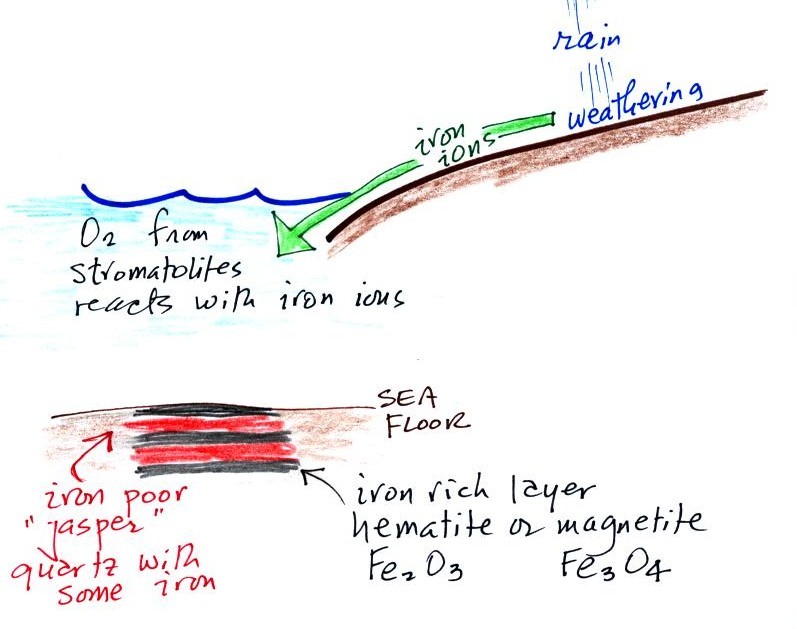
Periodically the oxygen production
would decrease or stop (rising
oxygen levels might have killed the cyanobacteria or seasonal changes
in incoming sunlight might have slowed the photosynthesis).
During these times of low
dissolved oxygen concentrations, layers of jasper would form on the
ocean bottom. Eventually the cyanobacteria would recover, begin
producing oxygen again, and a new layer of hematite or magnetite would
form. The rocks that resulted, containing alternating layers of
black hematite or magnetite and red layers of jasper are known as the
banded iron formation (Point 3). In addition to the
red
and black layers, the tiger
iron contains yellow layers made of fibers of quartz. The
rocks are fairly heavy because they contain a lot of iron, but the most
impressive thing about them in my opinion is
their age - they are a few billion years old! And thanks for
returning them by the way.
Eventually the dissolved iron in
the ocean was used up (Point 4
in the timeline figure above).
Oxygen produced by cyanobacteria no longer reacted with iron and was
free to move from the ocean into the
atmosphere. Once in the air, the oxygen could react with iron in
sediments on the earth's surface. This produced red colored
(rust colored) sedimentary rock. None of these socalled red beds
are older than
about 2 B years old. Thus it appears that a real buildup up
oxygen began around 2 B years ago. Oxygen concentrations reached levels
that are about the same as today around 500 to 600 years ago (Point 5
in the figure).
Now we're ready to start a section on air pollutants. We'll
spend the rest of today, Monday and Wednesday next week on this topic.
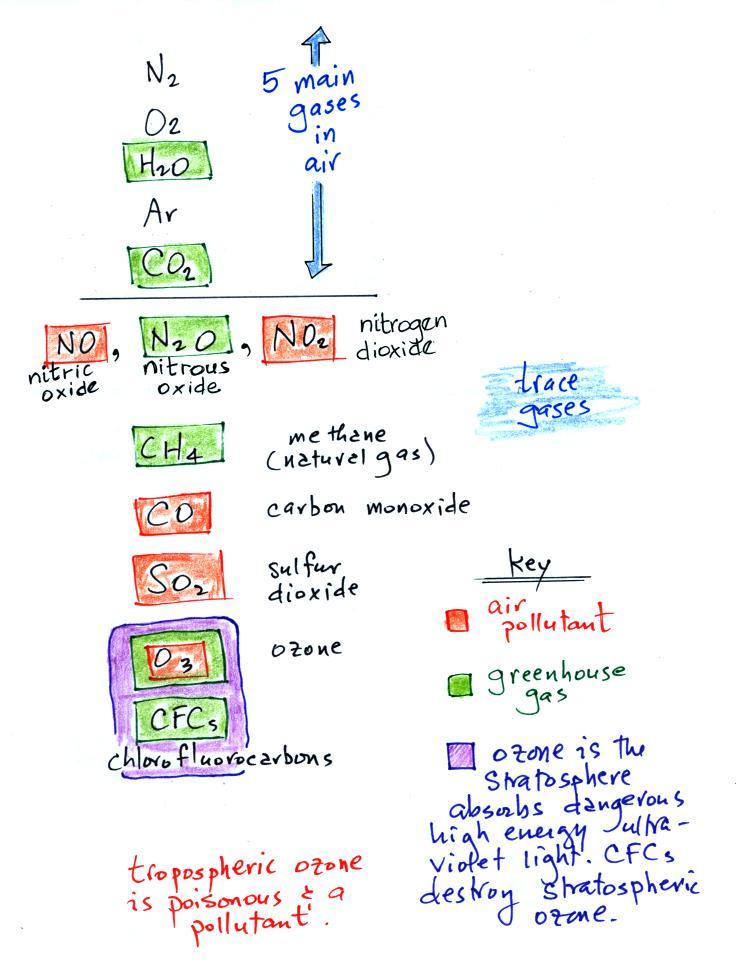
We listed
the 5 most abundant gases in the atmosphere at the beginning of
class.
Several more important trace gases were added to the
list in
class. Trace gases are gases found in low
concentrations (and often time the concentrations are variable).
Low concentrations doesn't mean they aren't
important, however.
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing
gas),
chlorofluorocarbons,
and
ozone
are
all
greenhouse
gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic and learn more about how the
greenhouse effect actually works later in the course.
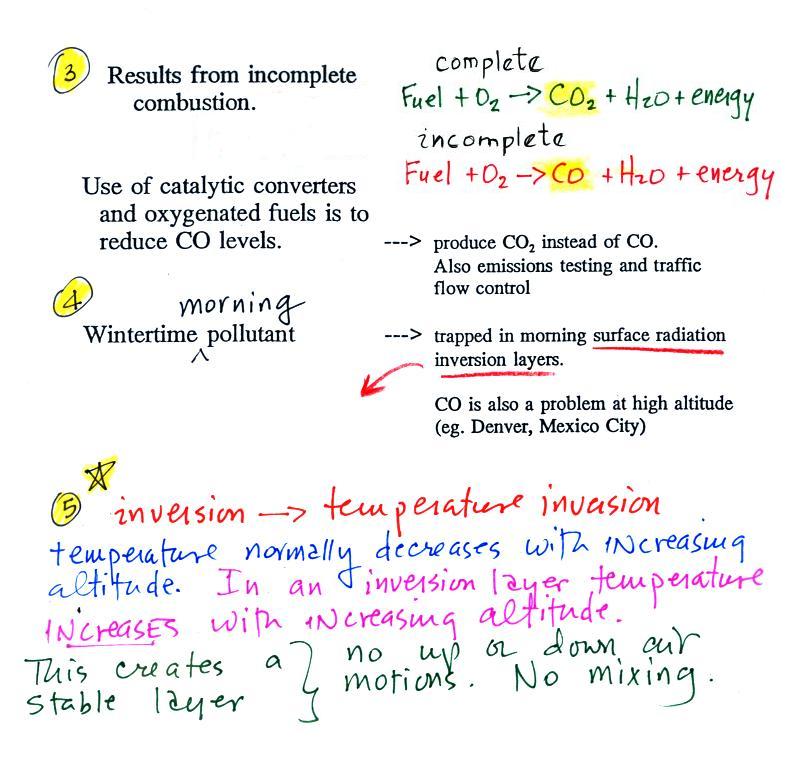
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and sulfur
dioxide are some of the major air pollutants. We'll cover some of
these in more detail today and early next week.
Ozone has sort of a Dr. Jeckyl and Mr. Hyde personality
(i) Ozone in the
stratosphere (a layer of the atmosphere between 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog.
(iii) Ozone is also a greenhouse gas.
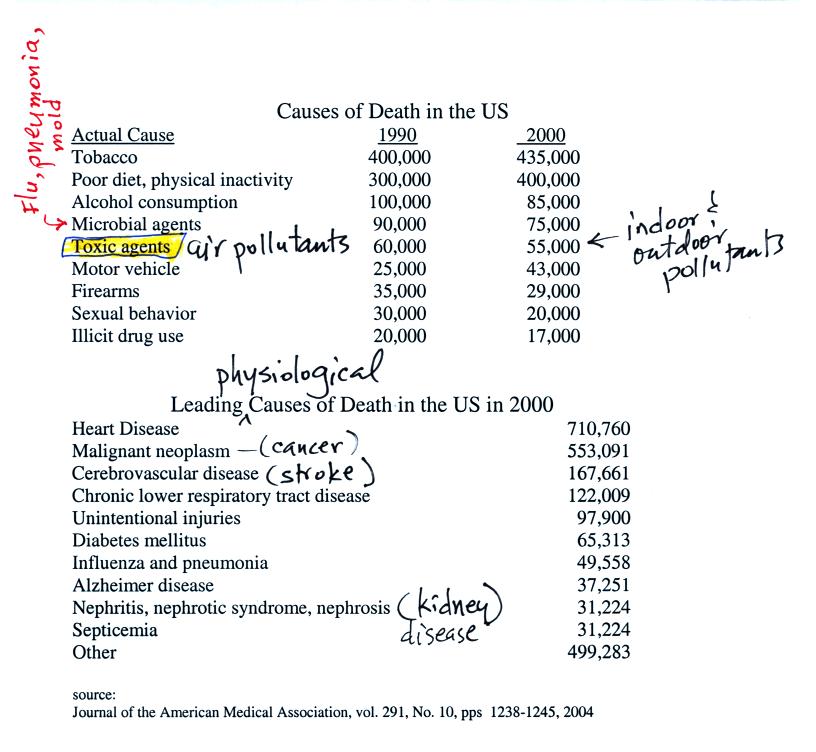
I like lists. Here are lists of the major causes of death in
the US and worldwide.
Air
Pollution is a serious health hazard in the US and around the
world. Click here
to download a copy of the statistics shown below.
Keep in mind that many of these
numbers are difficult to measure
and some may contain a great deal of uncertainty. The row that is
highlighted, toxic agents, contains estimates of deaths caused by
indoor and outdoor air pollution, water pollution, and exposure to
materials such as asbestos and lead both in the home and at the work
place. It is estimated that 60% of the deaths are due to exposure
to particulate matter, something that we will examine in a little more
detail next week.
Air pollution is a serious hazard
worldwide. Interestingly indoor air pollution is, in many places,
a more serious threat than outdoor air pollution. I'm not sure
how the researchers determine that 150,000 people are killed by climate
change every year.
The Blacksmith
Institute listed the Top 10 polluted places in the world in a
2007 report. The report has received a lot of worldwide
attention. If you go to this
address (click on 2007 at the top left edge of the page) you can
view the report online or download and print a
copy of the report. This is just in case you are interested.
We had time to start a section on carbon monoxide.
We'll finish this next Monday. You'll find
additional information on carbon monoxide and other air pollutants at
the Pima
County Department of
Environmental Quality website and also at the US Environmental
Protection Agency
website.
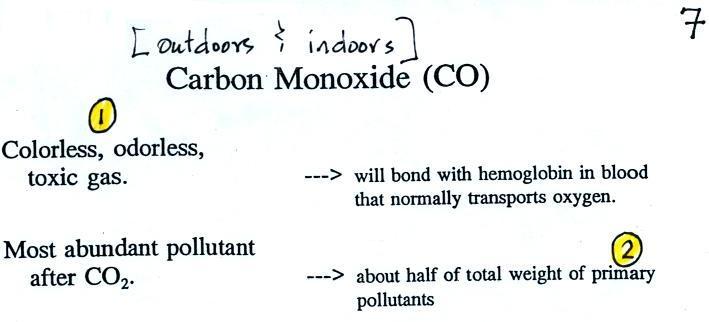
We will mostly be talking about
carbon
monoxide found outdoors, where it would rarely reach fatal
concentrations. Indoors is a serious hazard indoors also where it
can (and does) build up to deadly concentrations. (
several people were almost killed in Tucson last December)
Carbon monoxide is insidious, you can't smell it or see it
and it can kill you (Point 1).
Once
inhaled,
carbon
monoxide
molecules
bond
strongly
to
the
hemoglobin
molecules
in
blood
and
interfere
with
the
transport
of
oxygen
throughout
your
body.
The article above mentions that the CO poisoning victims
were put inside a hyperbaric (high pressure) chamber filled with pure
oxygen. This must force oxygen into the blood and displace the
carbon monoxide.
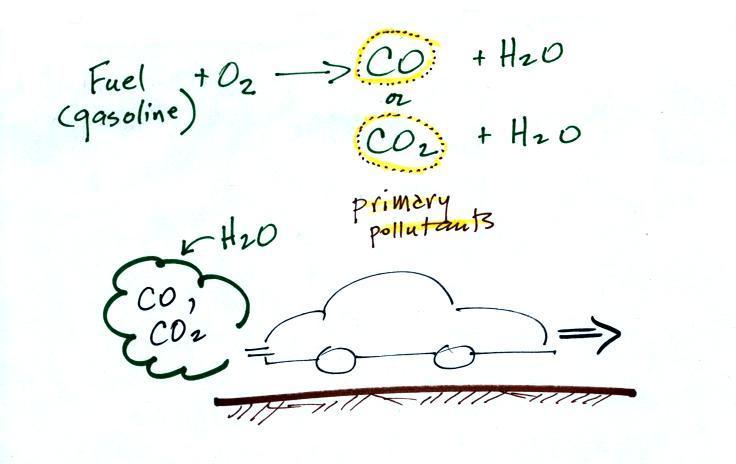
CO is a primary pollutant (Point 2
above). That means it goes
directly from a source into the air, CO is
emitted directly from an automobile tailpipe into the atmosphere for
example. The difference between
primary and secondary pollutants is probably explained
best in a series of pictures.
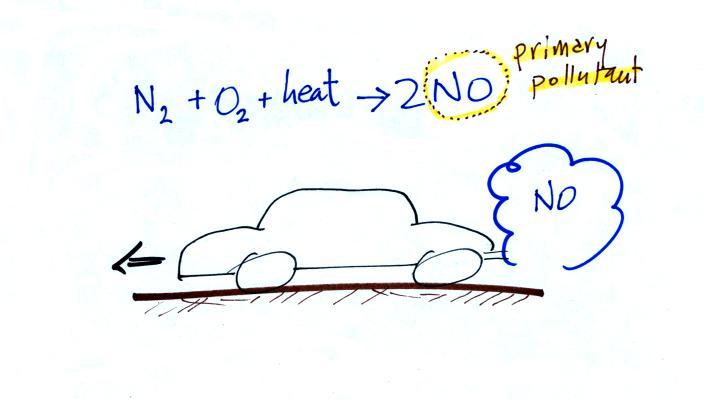
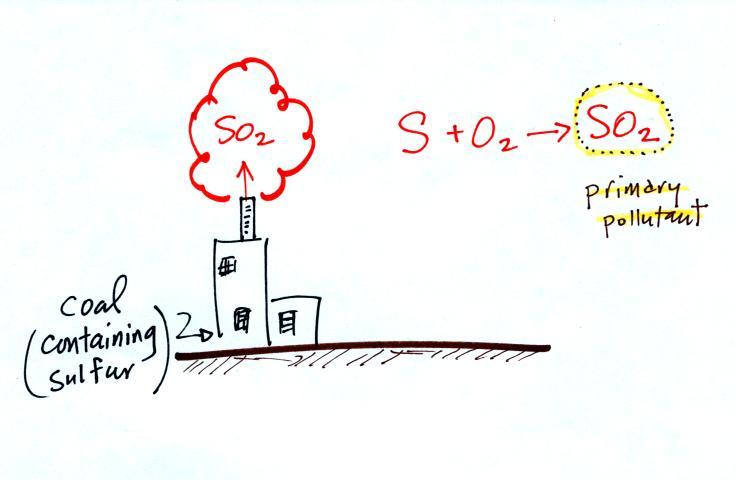
In addition to carbon monoxide, nitric oxide (NO) and sulfur
dioxide (SO2), are also primary
pollutants. They all go
directly from a source (automobile tailpipe or factory chimney) into
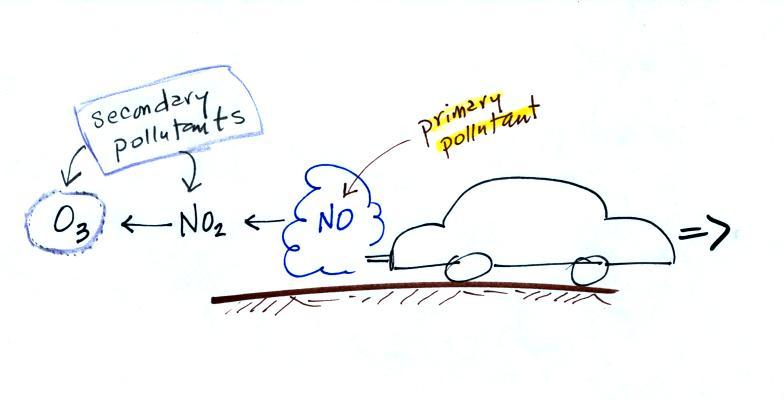
the atmosphere. Ozone is a
secondary pollutant (and here we are referring to tropospheric ozone,
not stratospheric ozone). It doesn't come directly from an
automobile tailpipe. It shows up in the atmosphere only
after a
primary pollutant has undergone a series of reactions.
Point 3
explains that CO is produced by incomplete
combustion of fossil
fuel (insufficient oxygen). Complete combustion would produce
carbon dioxide,
CO2. Cars and trucks
produce much of the CO in
the
atmosphere in Tucson.
Vehicles must now
be fitted with a catalytic
converter that will change CO into CO2
(and also NO into N2
and
O2
and hydrocarbons into H2O
and CO2).
In
Pima
County
vehicles
must
also
pass
an
emissions
test
every
year
and
special
formulations
of
gasoline
(oxygenated fuels) are used
during the winter months to try to reduce CO emissions.
In the atmosphere CO concentrations peak on winter
mornings (Point 4). The
reason for this is surface radiation inversion layers. They are
most likely to form on cold winter mornings.
In an inversion layer (Point 5)
air temperature
actually increases with increasing altitude which is just the opposite
of what we are used to. This produces stable atmospheric
conditions which means there is little up or down air motion.
There is very little vertical
mixing in a stable air
layer.
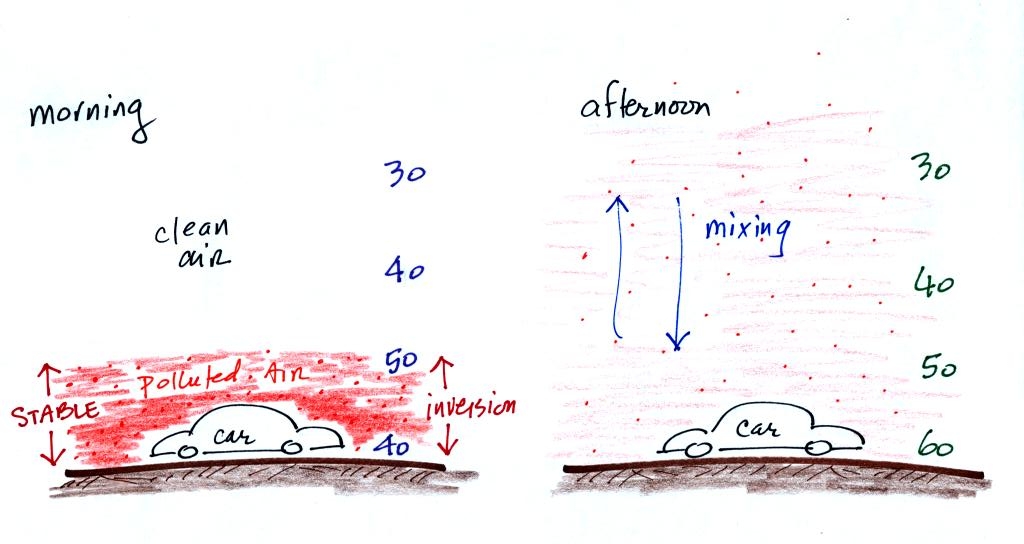
In the left figure above, notice how temperature increases from 40
F to 50 F in the thin air layer next to the ground (it then decreases
with altitude above that). This is the stable inversion
layer. When CO is emitted into the thin
stable layer, the CO
remains in the layer and doesn't mix with cleaner air above. CO
concentrations build.
In the afternoon, the ground warms, and the atmosphere becomes
more
unstable. Temperatures decrease with increasing altitude in the
right figure above. CO emitted
into air at the surface mixes with cleaner air above. The CO
concentrations are effectively diluted.
Thunderstorms
contain strong up
(updraft) and down (downdraft) air motions. Thunderstorms are a
sure indication of unstable
atmospheric conditions.