Acid Rain Demonstration
Some common acids are listed below. In solution the acid
molecules dissociate (split). The presence of H+ ions
is what
makes these materials acids.
Actually
for a solution to be acidic it must have an H+ ion
concentration that
is greater than the H+ ion concentration found in distilled
water. The H+ ion concentration in pure water is 10-7
moles of H+
ions per liter of water. We often use the pH scale to measure
acid concentration. An H+ ion concentration of 10-7
moles/liter
corresponds to pH 7.
A basic solution will have an H+ ion concentration that is
lower than
found in pure water.
Now we can
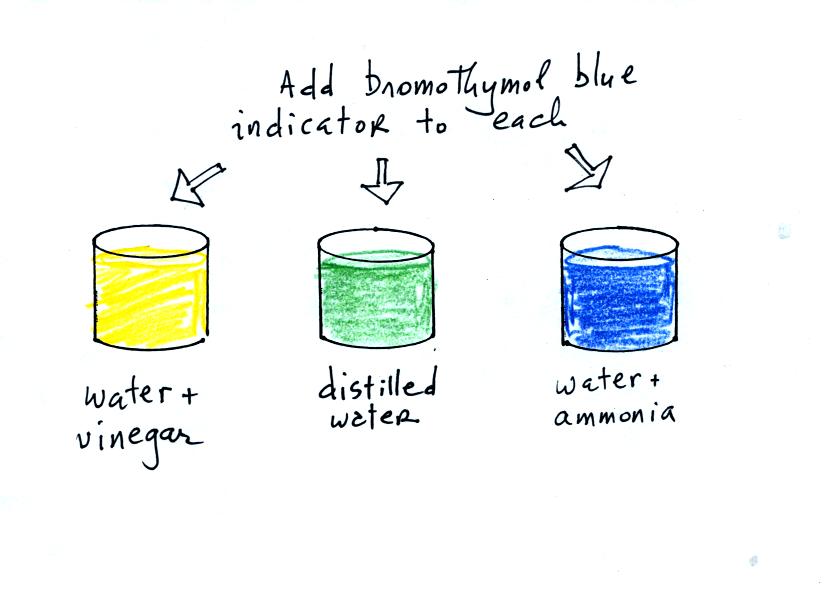
proceed to the demonstration. We will start with three 1000 mL
beakers. They have all been filled with distilled water.
Some vinegar (contains acetic acid) has been added to the left beaker.
Some ammonia (a base) has been added to the right beaker.
Then we add some bromothymol blue color indicator solution to all three
beakers. Bromothymol blue has the amazing property of changing
color depending on whether it is mixed with an acid or a base.

Dry ice sublimates. It turns directly from solid to ice (ordinary
ice melts and turns from solid to liquid). The gaseous CO2
is
invisible but you can tell it is there because of the bubbles in the
tap water. Some of the CO2 dissolves as it bubbles
through the
water and slowly turns the water acidic. You can tell that this
is occurring because the bromothymol blue indicator turns from deep
blue to green and eventually to yellow.

While we didn't actually produce acid rain, there is concern that
increasing atmospheric concentrations of carbon dioxide will dissolve
and acidify the world's oceans. This is discussed in the
following article from The Christian Science Monitor. You can
download a copy of the article here.
The main concern over increasing atmospheric carbon dioxide
concentrations is global warming from enhancement of the greenhouse
effect. We will discuss this topic in a few weeks.






