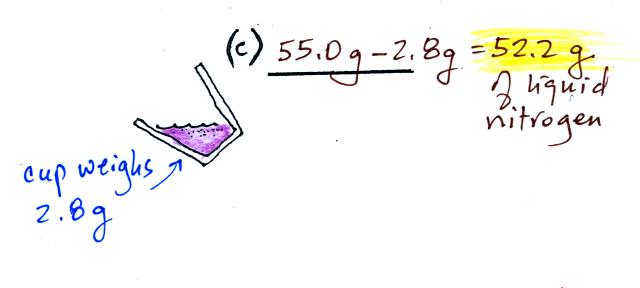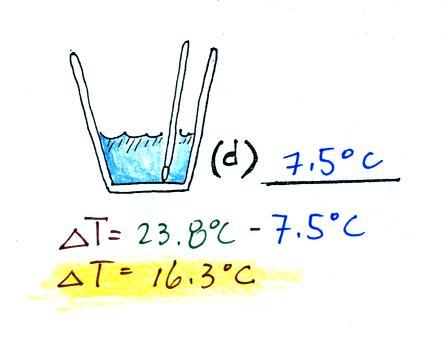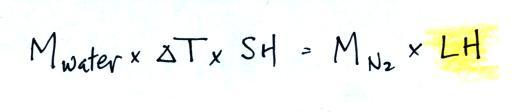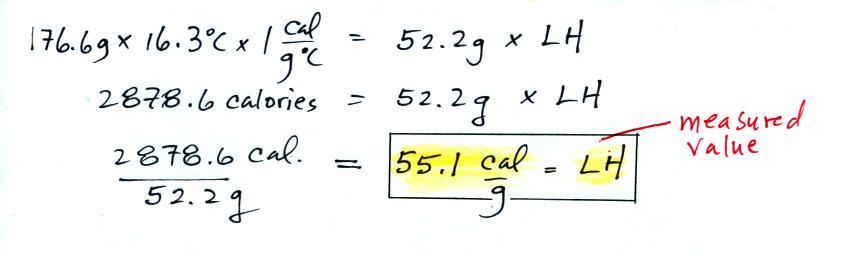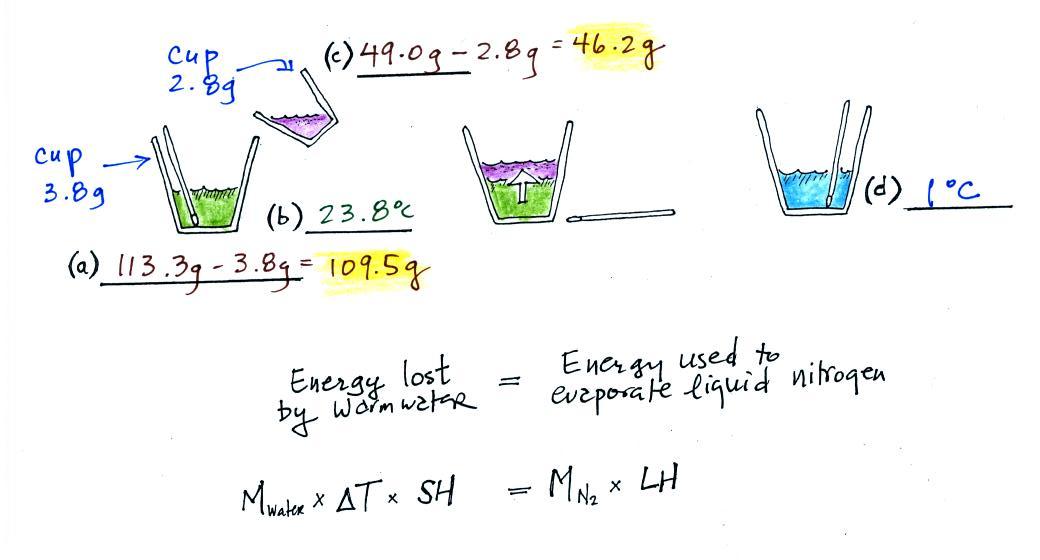Thursday Sep. 29, 2011
click here
to download today's notes in a more printer friendly format
A couple of songs from Dire Straits this morning ( "Brothers in Arms"
and
"Down
to the Waterline")
After a monumental effort, the Expt. #1 reports have been graded and
were returned in class today. You are allowed to
revise your reports if you want
to (you don't have to). The revised reports will be due in 2
weeks, on or before Thu., Oct. 13 (the date of Quiz #2). You only
need to rewrite
portions where you want to earn additional credit. Please return
your original report with your revised report.
And to encourage people to read through the Online Notes, I'm
likely to bury a link to an Optional Assignment or maybe embedding
questions in the Online Notes. So keep an eye out for that.
During the next couple of weeks we
will be moving into a completely different topic and will be concerned
with energy,
temperature, heat, energy transport, and
energy
balance between the earth, atmosphere, and space.
It is easy to
lose sight of the main concepts because there are so many
details. The following (found on pps 43&44 in the photocopied
Class Notes) is meant to introduce some of what we will be covering in
class. (some of the figures that follow are from a previous
semester and
may differ somewhat from what we did in class)
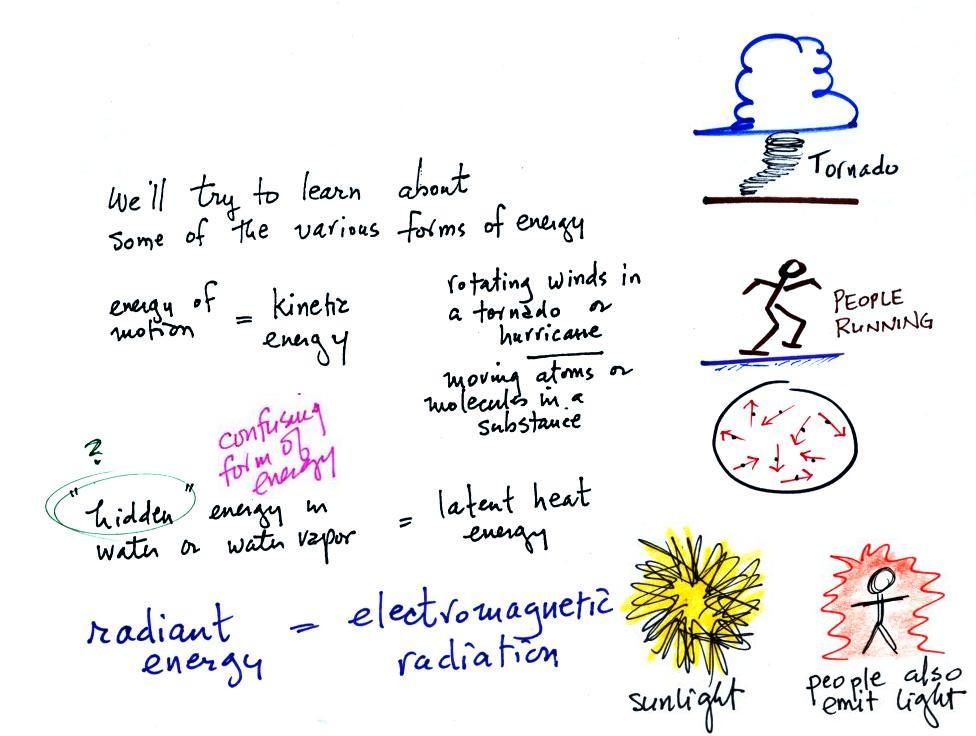
Types
of
energy
We will learn the names of several different types or forms of
energy. Kinetic energy is energy of motion. Some examples (both large
and microscopic scale) are mentioned
and sketched above. This is a relatively easy to visualize and
understand form of energy.
Latent heat energy is perhaps the most underappreciated and most
confusing type of energy. The word latent refers to energy that is
hidden in water and water vapor. The hidden energy emerges when
water vapor condenses or water freezes (the energy had been added
earlier when ice was melted or water was evaporated).
Radiant energy is a very important form of energy that was for
some
reason left off the original list. Sunlight is an example of
radiant energy that we can see and feel (you feel warm when you stand
in sunlight). There are many types of radiant energy
that are invisible (such as the infrared light that people emit).
Electromagnetic radiation is another name for
radiant energy.
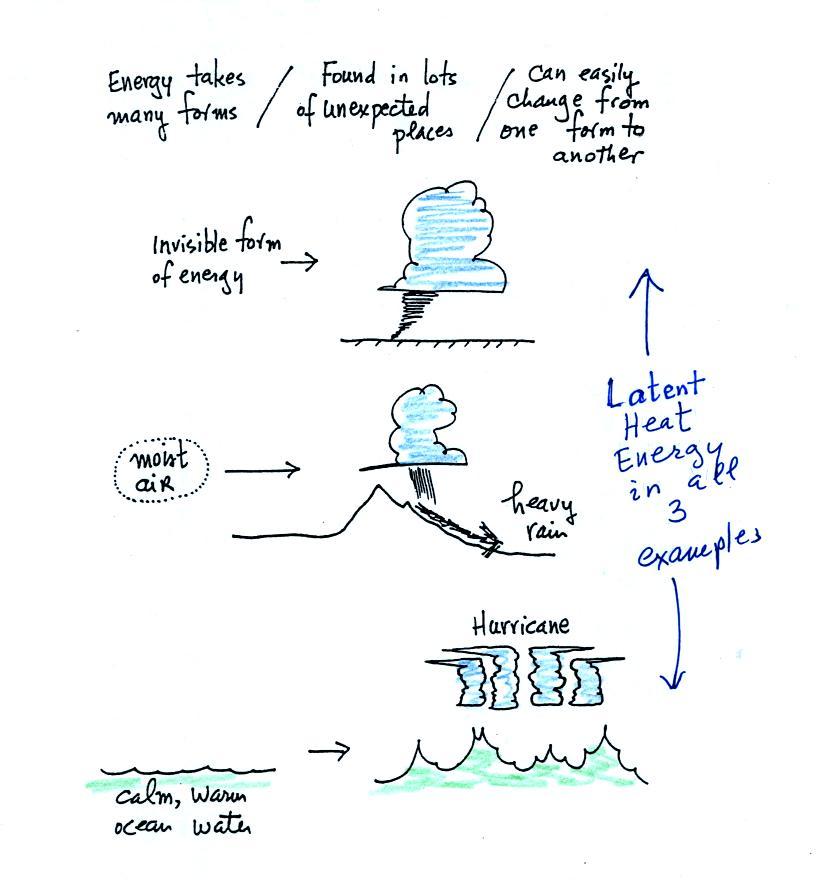
Water vapor is effectively a particularly important form of
invisible
energy.
When water vapor condenses to produce the water droplets (or ice
crystals) in a
cloud, an enormous amount of latent heat energy is released into the
atmosphere. This then might get turned into the violent winds in
a tornado or a hurricane.
It is hard to visualize or appreciate the amount of energy
released
into the
atmosphere during condensation. You can imagine the work that you
would need to do to carry a gallon of water
(8 pounds) from Tucson to the top of Mt. Lemmon. To
accomplish
the same thing Mother Nature must first evaporate the water and (if my
calculations are correct) that requires about 100 times the energy that
you would use to carry the 8 pounds of water to the summit of Mt.
Lemmon. And Mother Nature transports a lot more than just a
single gallon.
Hurricanes derive their energy from the heat in warm ocean water
and from latent heat in water vapor that condenses while forming the
clouds of the hurricane.
Energy transport
Four energy transport
processes are listed below.
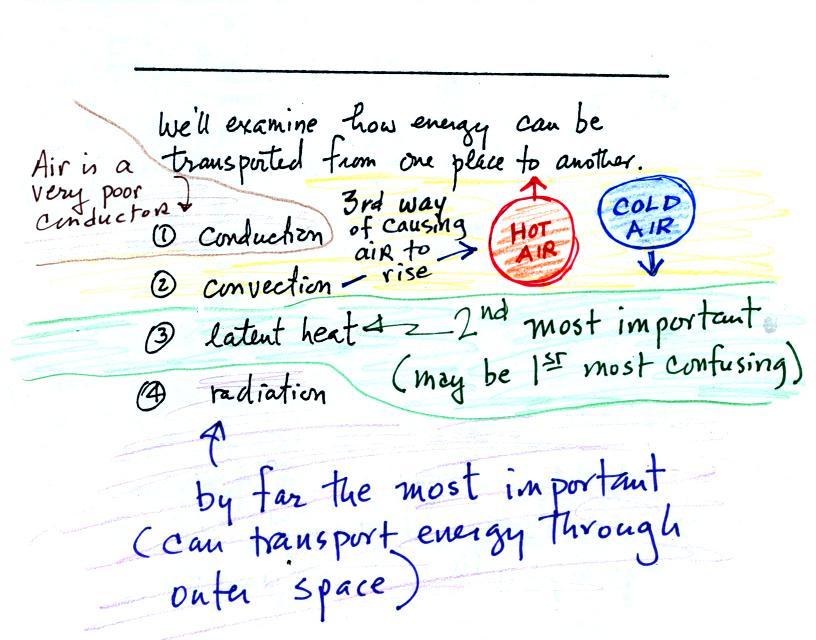
By far the
most important process is energy transport in the form of
electromagnetic radiation (sunlight is a common
form of electromagnetic radiation). This is the
only process that can transport energy through empty space.
Electromagnetic radiation travels both to the earth (from the sun) and
away from the earth back into space. Electromagnetic radiation is
also
responsible for about 80% of the
energy transported between the ground and atmosphere.
You might be
surprised to learn that latent heat is the second most important
transport process.
Rising parcels of warm air and sinking parcels of cold air are
examples of free convection. Because of convection you feel
colder or
a cold windy day than on a cold calm day.
Ocean currents are also an example of convection. Ocean currents
transport energy from the warm tropics to colder polar regions. Not all of these
details were mentioned in class.
Convection is a 3rd way of causing rising air
motions in the atmosphere (convergence
into centers of low pressure and fronts are other 2 ways we've
encountered so far)
Conduction is the least important energy transport at least in the
atmosphere. Air is such a poor conductor of energy that it is
generally considered to be an insulator.
Energy
balance
and the
atmospheric greenhouse effect
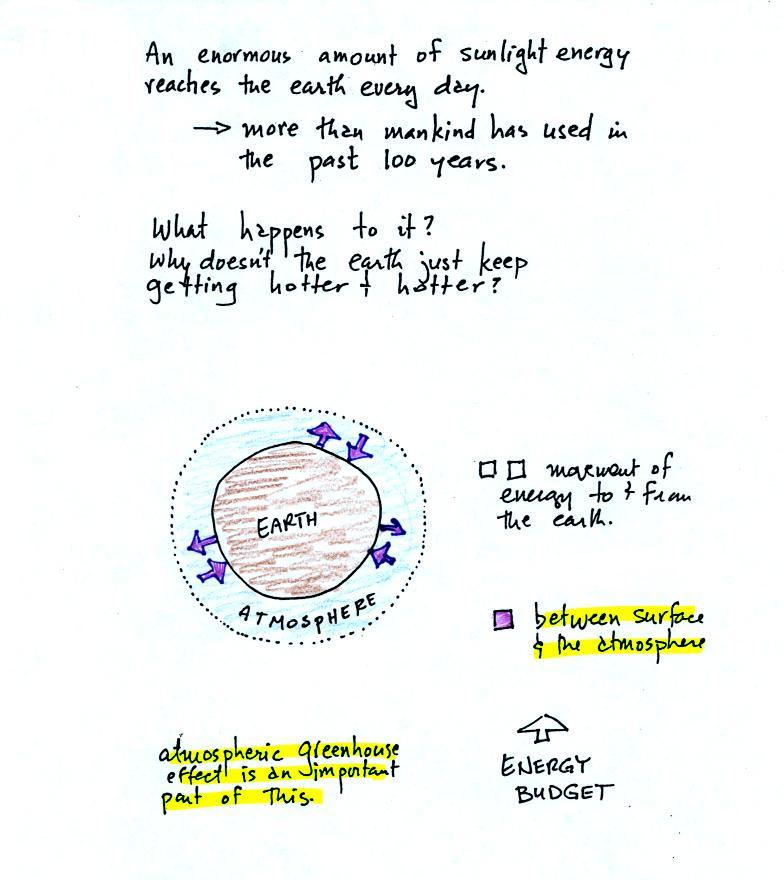
The next picture (the figure drawn in class has been split into three
parts for improved clarity) shows energy being transported from the sun
to
the earth in the form of electromagnetic radiation.

We are aware of this energy because
we can see it (sunlight
also contains invisible forms of light) and feel it. With all of
this energy arriving at and
being
absorbed by the earth, what keeps the earth from getting hotter and
hotter? If you park your car in the sun it will heat up.
But there is a limit to how hot it will get. Why is that?
It might be helpful when talking about energy balance to think of a
bank account. If you periodically deposit money into your account
why doesn't the balance just grow without limit. The answer is
that you also take money out of the account and spend it. The
same is true of energy and the earth. The earth absorbs incoming
sunlight energy but also emits energy back into
space (the orange and pink arrows in the figure below)
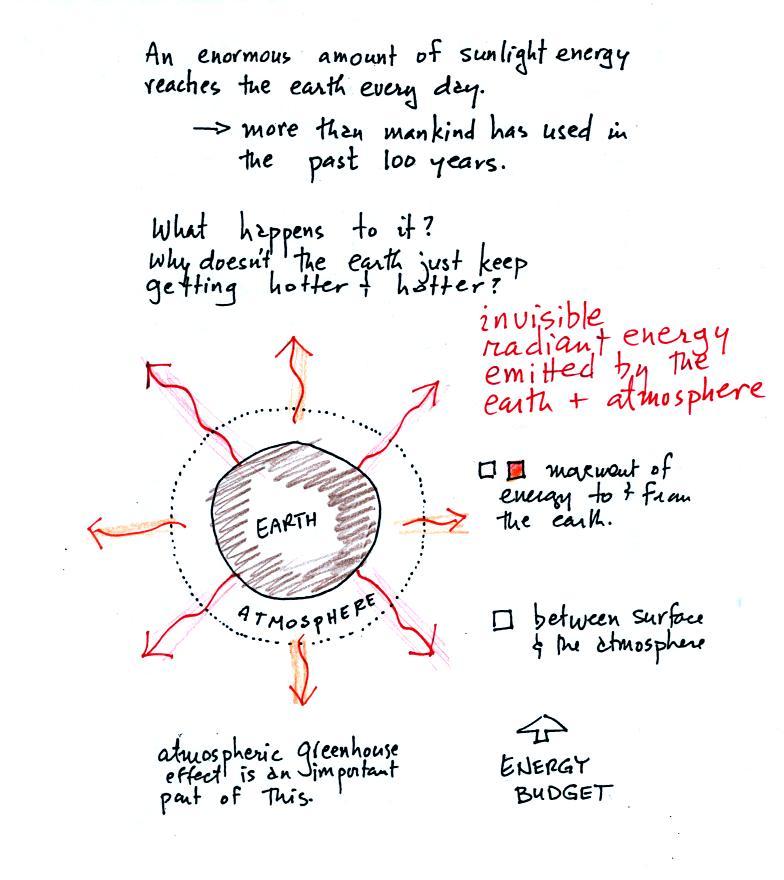
Energy is emitted in the form of
infrared light is an
invisible form of energy (it is weak enough that we
don't usually feel it either). A balance
between incoming and outgoing energy is achieved and the earth's annual
average temperature remains constant.
We will also look closely at energy transport between the earth's
surface and the atmosphere. This is where latent heat energy transport,
convection and conduction operate (they can't transport energy beyond
the atmosphere and into outer space).
That is also where the atmospheric
greenhouse functions. That will be a important goal -
to
better understand how the atmospheric greenhouse effect works.
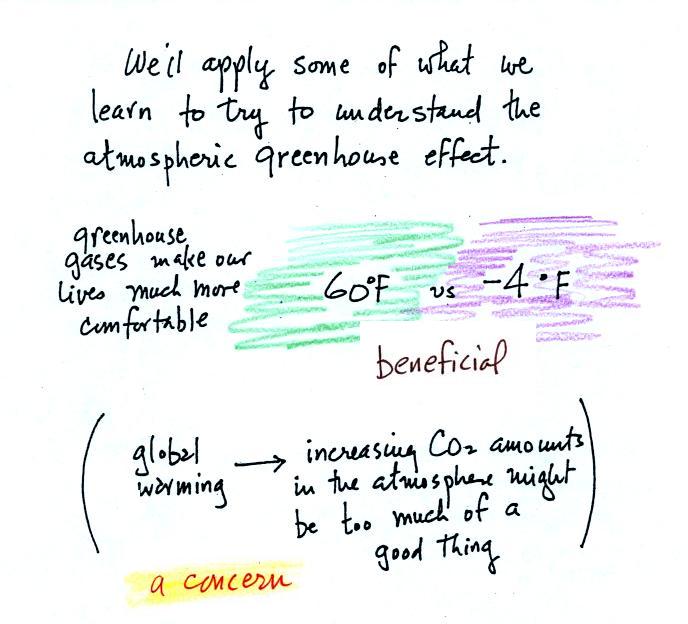
The greenhouse effect is getting a
lot of "bad press". If the earth's atmosphere didn't contain
greenhouse gases and if there weren't a greenhouse effect, the global
annual average surface temperature would be about 0 F (scratch out -4 F
and put 0 F, it's easier to remember). Greenhouse gases raise
this
average to about 60 F and make the earth a much more habitable
place. That is the beneficial side of the
greenhouse effect.
The detrimental side is that atmospheric greenhouse gas concentrations
are increasing. This might enhance or strengthen the greenhouse
effect and
cause the earth to warm. While that doesn't necessarily sound bad
it could have many unpleasant side effects. That's a subject
we'll explore briefly later in the semester.
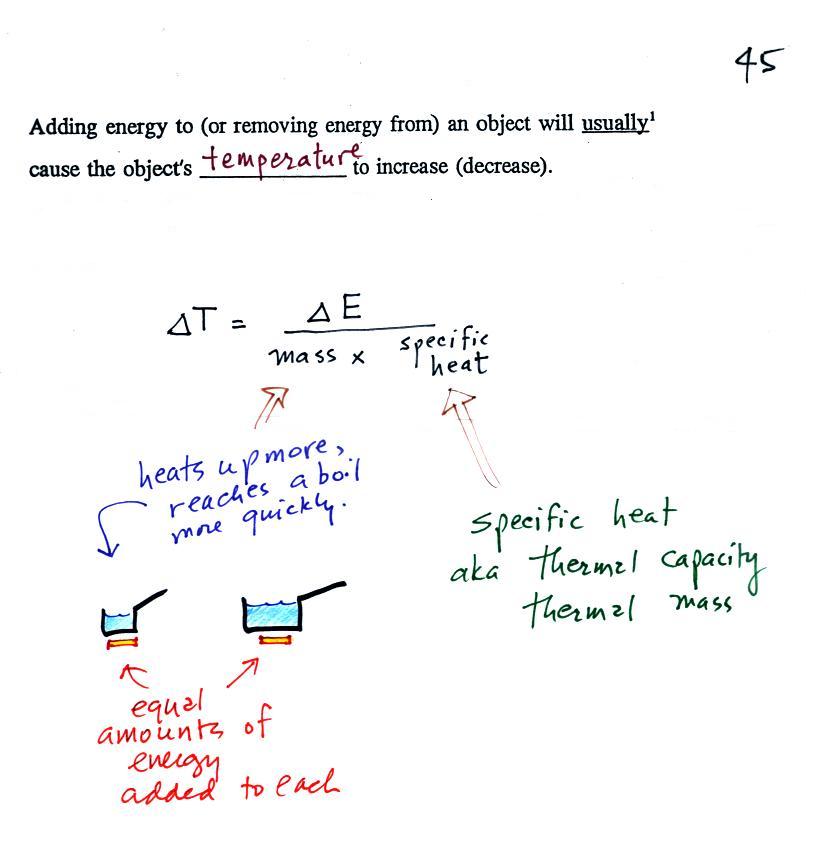
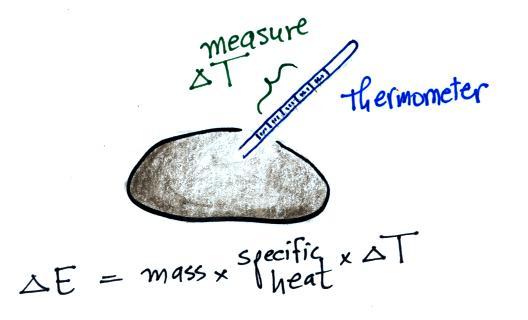
When you
add energy to an object, the object will usually
warm
up (conversely when you take energy from an object the object will
cool). It is relatively easy to come up with an equation that
allows
you to figure out what the temperature change will be (one of those
equations I'll probably write on the board during the next quiz if you
ask me to - try
to understand it, you don't have to memorize it).
The temperature change, ΔT, will
first depend on
how much energy was added, ΔE. This is a
direct proportionality, so ΔE is in the
numerator of the
equation (ΔE and ΔT are
both positive when energy is added,
negative when energy is removed)
When you add equal amounts of energy to large and small pans
of water, the small pan will heat up more
quickly. The temperature change, ΔT, will
depend on the
amount of water, the mass. A small mass will mean a large ΔT,
so
mass
should
go
in the denominator of the equation.
Different materials
react differently when energy is added to them. A material with a
large specific heat will warm more slowly than a material with a small
specific heat. Specific heat has the same kind of effect on ΔT
as
mass. Specific heat is sometimes called "thermal mass" or
"thermal capacity." You can think of specific heat as
being thermal inertia - a substance with high specific heat, lots of
thermal inertia, will be reluctant to change temperature.
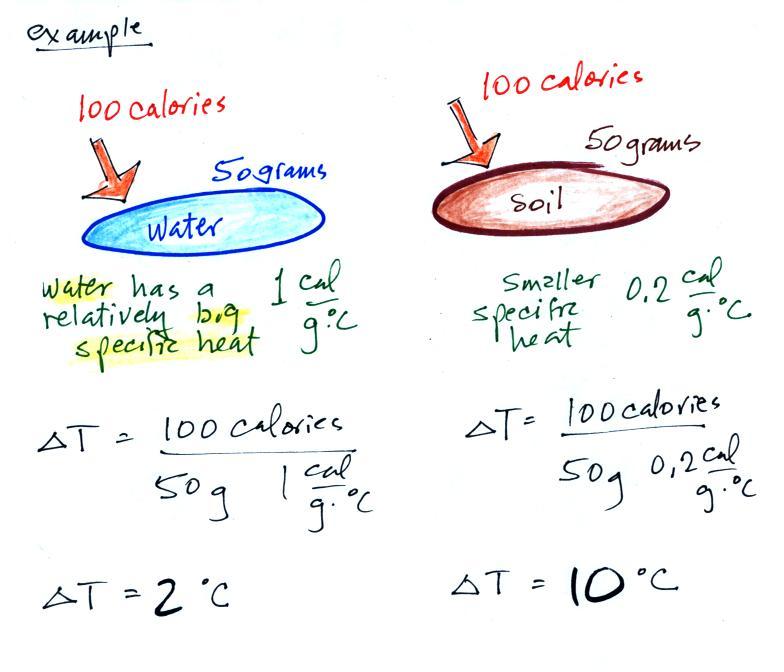
Here's an important example that will show the effect of specific
heat (middle of p. 45)
Equal
amounts of energy (100 calories, note that calories are units of
energy) are added to
equal masses (50 grams) of water and soil. We use water and soil
in the
example because most of the earth's surface is either ocean or land.
Water has a higher specific heat than soil, it only warms up 2o
C.
The soil has a lower specific heat and warms up 10o C, 5
times more
than the water (the specific heat of the soil is 5 times smaller than
that of water).
These different rates of warming of water and soil have
important effects on regional climate.
Oceans moderate the climate.
Cities near a large body
of water won't warm as much in the summer and won't cool as much during
the winter compared to a city that is surrounded by land.
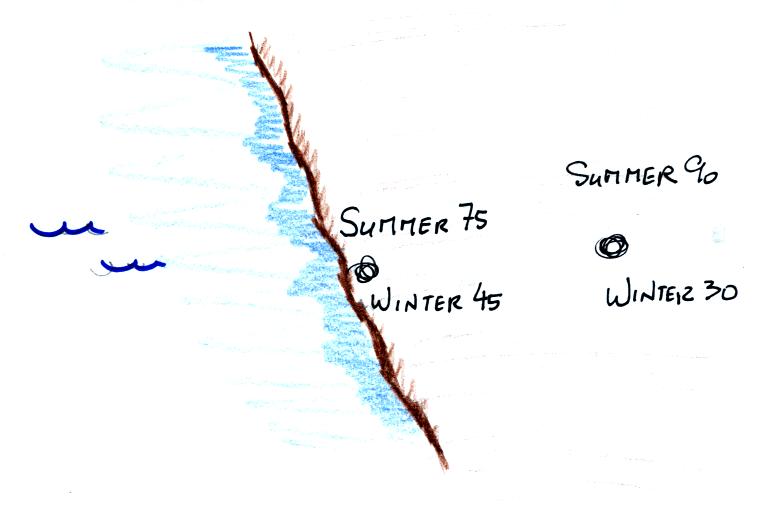
The yearly high and low monthly average temperatures are shown at
two locations above. The city on the
coast has a 30o F annual range of temperature (range is the
difference between the summer and winter temperatures). The
city further
inland (assumed to be at the same latitude and altitude) has an annual
range of 60o F. Note that both cities have the same 60o
F annual
average temperature. We'll see a much more dramatic example of
the moderating effect of water on climate in a couple of weeks.
Here's another situation where you
can take advantage of water's
high specific heat to moderate "micro climate."
If this were the Spring semester I
would probably already have planted some tomatoes in my
vegetable garden. The start of my summer vegetable garden. It
still
gets
plenty
cold
enough at night in February or early March to kill tomatoes
(the brocolli
and lettuce in the background can handle a
light frost) so you have to protect the them.

Here's one way of doing that.
You
can
surround
each
plant
with
a
"wall
of
water"
- a tent
like arrangement that surrounds each plant. The cylinders are
filled with water and they take advantage of
the high specific
heat of water and won't cool as much as the air or soil would during a
cold
night. The walls of water produce a warm moist microclimate that
the tomato seedlings love.
This being the Fall semester I will probably be planting my winter
vegetable garden soon, maybe even this coming weekend. I mention
it because if something bad happens to the young seedlings I'm likely
to get very mad. If you hear of a freak hailstorm, torrential
rains, or something like that it might be a good idea to sit in the
back of the classroom until you see what kind of a mood I'm in.
Adding
energy to an object will usually cause it to warm. But there
is another possibility (bottom p. 45), the object could change
phase (change
from solid to liquid or
gas). Adding energy to ice might cause
the
ice to melt. Adding energy to water could cause it to
evaporate.
The equation at the bottom of the
figure above allows you to
calculate how much energy is required to melt ice or evaporate water or
sublimate dry ice. You multiply the mass by the latent heat, a
variable that depends on the particular material that is changing
phase. The latent heat of vaporization (evaporation) is the
energy required to evaporate 1 gram of a material.
If you add energy to or remove
energy from an object, the
object
will usually change temperature. You can calculate the
temperature change if you know the object's mass and its specific
heat. That's the equation we used in the example calculation
above. It's shown again below.

We conducted an experiment in the
last part of the class and we needed to be able to measure ΔE.
It's not that hard to do. If you are able to measure
the ΔT that occurs when energy is added to or
removed from an object we can write the same equation in slightly
different form and use it (and the measurements of ΔT)
to
compute
ΔE.
A couple of groups of students from
the class were nice enough to volunteer
to
perform the experiment.
The students that are doing Experiment #2 are doing something
similar, they are measuring the latent heat
of fusion of ice, the energy needed to melt one gram of ice.
You'll
find the following figure on p. 45a in the photocopied
Classnotes. This is pretty confusing even after I neatened it up
a little bit after class. And to make matters even worse, I think I made a
couple of calculation mistakes when we were working through
this. I'll point them out as we work through this step by step
below.
So here's a step by step
explanation of what the students did:
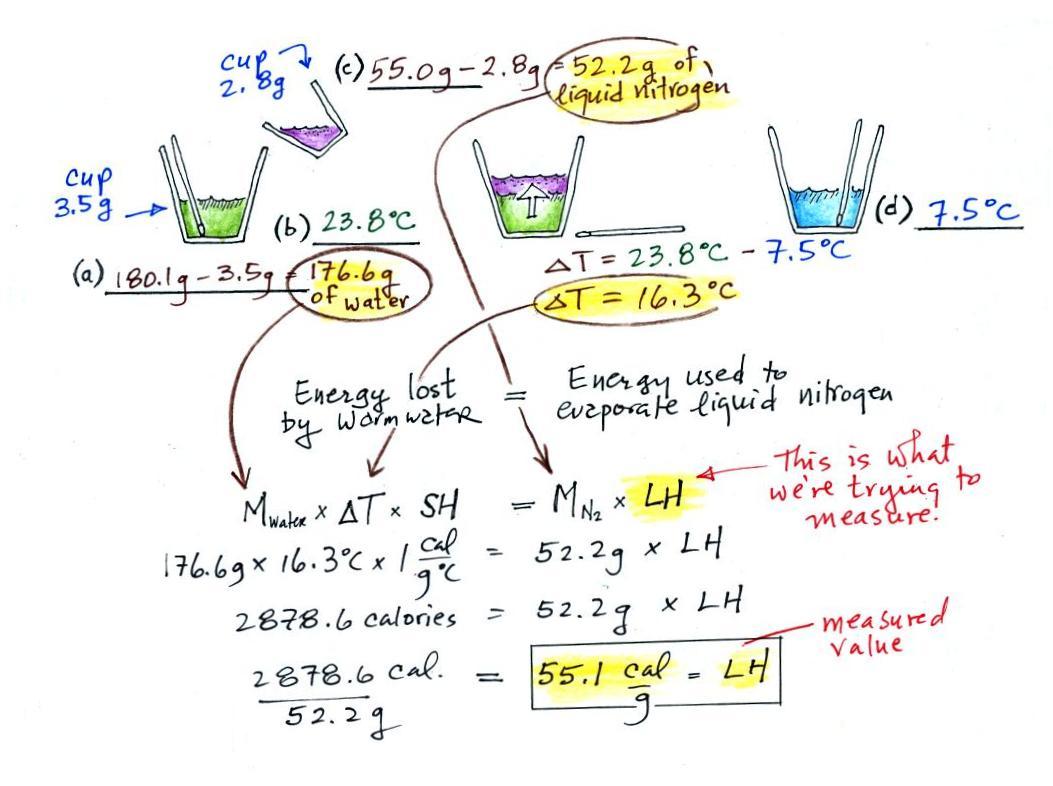
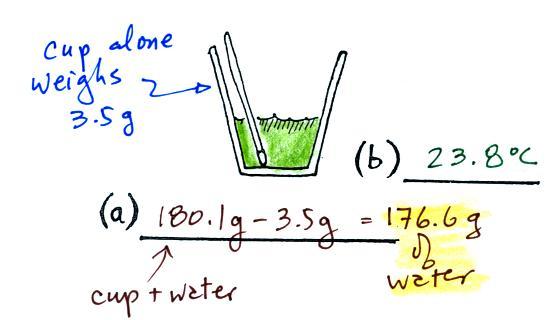
(a)
Some room temperature water poured into a styrofoam cup weighed
180.1g. The cup itself weighed 3.5 g, so they had 176.6 g of
water.
(b)
The water's temperature was measured with the thermometer and was
23.8 C (room temperature).
(c)
Some liquid nitrogen was poured into a second smaller styrofoam
cup. That weighed 55.0 g. Subtracting the 2.8 g weight of
the cup means we had 52.2 g of liquid nitrogen. Here's the first
mistake: I'm pretty sure I forgot to subtract the 2.8 g while
working through this in class.
We don't need to measure the temperature of the liquid nitrogen
(doing so would probably destroy the thermometer). It had already
warmed as much as it can ( to -320 F or something like that). Any
additional energy added to the liquid nitrogen will cause it to
evaporate.
(d)
After the liquid nitrogen had evaporated the water's
temperature was remeasured. It had dropped to 7.5 C.
We started out with water that was 23.8.0 C, so that is a temperature
drop of 16.3 C.
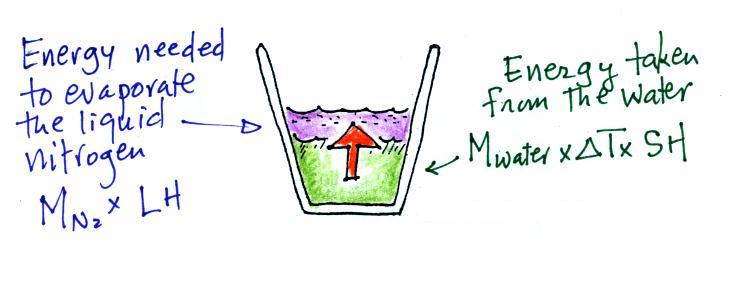
It takes energy to turn liquid nitrogen into nitrogen gas.
The energy needed will be taken from the water (the red arrow below,
energy naturally flows from hot to cold).
Because the experiment was performed in an insulated sytrofoam cup we
will assume all of the energy taken from the water is used to evaporate
nitrogen. No energy flows into the room air or anything like
that. We will set the two equations above equal to each
other. This is an energy balance equation.
We know the mass of the nitrogen (45.8 g) and the water (172.3
g). We measured the ΔT (14.5 C) and we
know the specific heat of water (1 cal/g C). We substitute them
into the equation above and solve for LH, the latent heat of
vaporization of liquid nitrogen. Here are the details of the
calculation:
This is
where I found the 2nd mistake. I wrote down 2875.6
calories in class (probably misread the number in the dim light at the
front of the room).
A
responsible & trustworthy student in
the class (though not a Buddhist monk) informed us that
the known value is 48 cal/g, so this measurement
was pretty close to the known value.
A second group of students also conducted an independent
measurement of LH. Their data are shown above, see if
you can calculate LH. Once you've given it a try (and
only after you've spent some time on it) click here
to see if
you did the calculation correctly.
Here's the equation again that allows you
to determine how much of a temperature change will occur when energy is
added to or removed from an object.
What exactly is happening
inside an object when it's temperature changes?
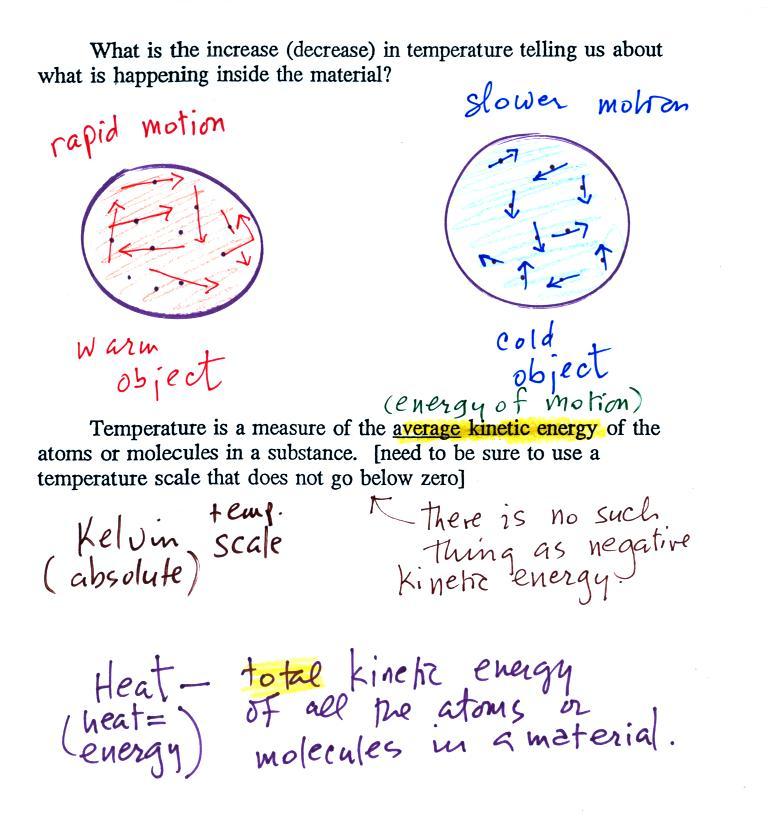
The figure above is on p. 46 in the
photocopied Class
Notes. Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material. Kinetic energy is energy of motion, so
the atoms or molecules in a cold
material will be moving more slowly than the atoms or molecules in a
warmer object.
You need to be careful what temperature scale you use
when
using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero (0 K is known as absolute zero). The smallest kinetic
energy you can have is zero
kinetic energy. There is no such thing as negative kinetic energy.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
Speaking of temperature scales,
there are three of them that you should be familiar with.
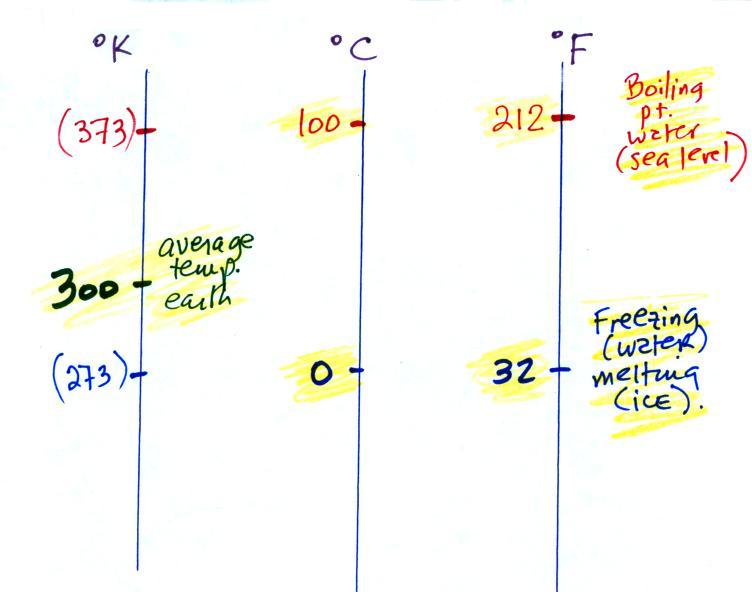
You should remember the
temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and perhaps the Kelvin
scale if you want to. 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth. That's a number you should remember.
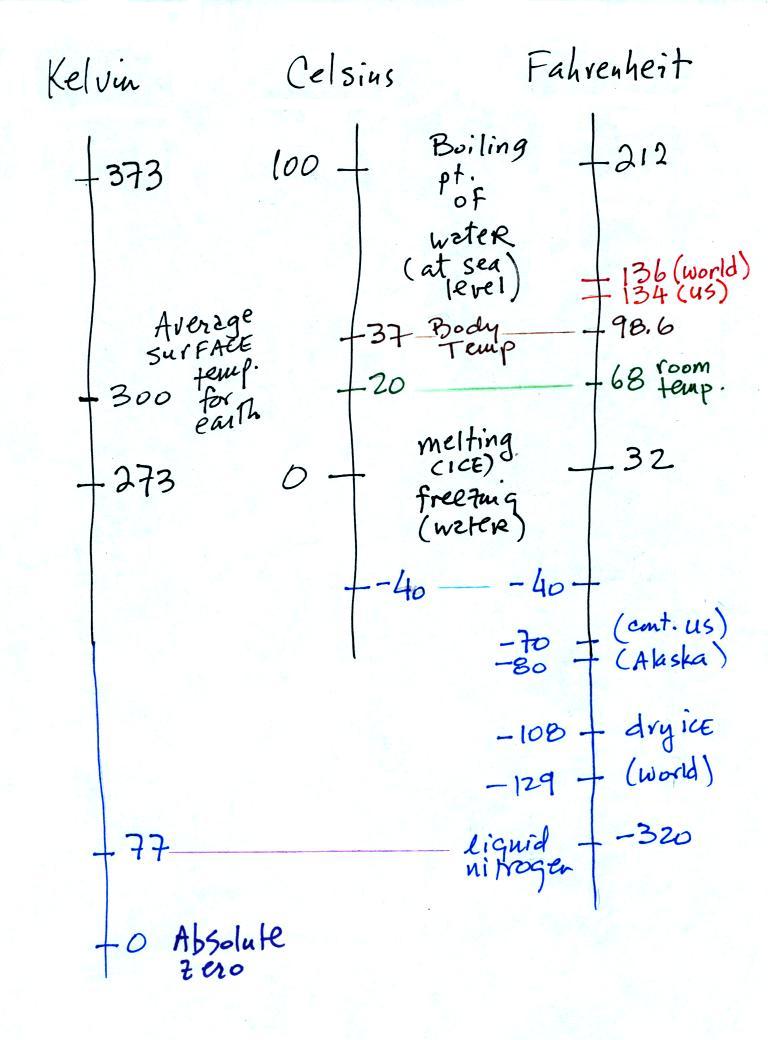
You certainly don't need to try to
remember all these
numbers. They're mostly just for informational purposes and to
help with questions on hidden optional
assignments. The world high temperature record was set in
Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than being
near or
surrounded by ocean (water moderates climate).
Liquid
nitrogen is cold but it is still quite a bit warmer than absolute
zero. Liquid helium gets within a few degrees of absolute zero,
but it's expensive and there's only a limited amount of helium
available. So I would feel guilty bringing some to class.
This next figure might make clearer the difference between
temperature (average kinetic energy) and heat (total kinetic energy).
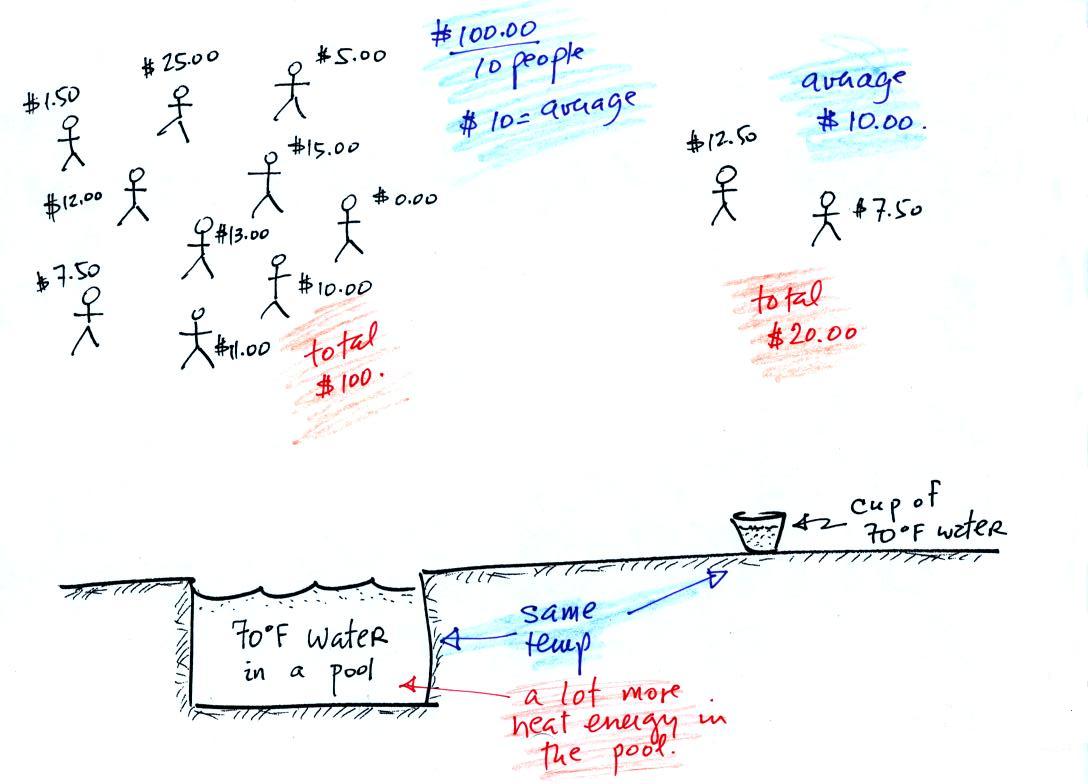
A cup of water and a pool of water
both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
water molecules in the pool than in the cup. So if you add
together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger total number than if you sum the kinetic energies of the
molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people shown have the same
$10 average
amount
of money per person (that's analogous to temperature). The $100
held by the larger group at the
left is
greater than the $20 total possessed by the smaller group of people on
the right (total amount of money is analogous to heat).