Monday Oct. 8, 2012
click here to
download today's notes in a more printer friendly format
A busy 10 minutes before the start of class with Green Cards to
distribute, Experiment #2 reports and materials to collect, a
demonstration to set up, and music playing in the background. You
heard "Swing 49", "Gypsymania", and "Turkish Delights" from the Live at
Birdland Django Reinhart NY Festival. I wasn't able to find any
of those songs on YouTube, but here is "Caravan" which
is a good substitute.
The Upper Level Charts Assignment has been graded. If you lost 3
pts or less on the assignment you earned a Green Card. Everyone
that turned in an assigment earned 0.5 pts of extra credit.
The last of the 1S1P Assignment #1 reports (the radon topic) has been
graded and was returned in class.
The final version of the Quiz #2 Study Guide
is now online.
The world would not look the same if we were able to see IR
light instead of visible light.
The picture at left was taken using normal film, film that is
sensitive to visible light. The picture at right used infrared
film. In both pictures we are looking at sunlight that strikes
the tree or the ground and is reflected toward the camera where it can
be photographed (i.e. these aren't photographs of light emitted by the
tree or the ground). The tree at left is green and relatively
dark (it reflects green light but absorbs the other colors of visible
light). The tree at right and the ground are white, almost like
they were covered with snow. The tree and grass on the ground are
reflecting infrared light. Here are many
more
images taken with infrared film.

Here's another example, photographs of the ground taken from an
air plane using ordinary film at left
(responds to visible light) and infrared film at
right. Notice how the IR photograph is able to "see through"
the
haze. The haze at left is scattered light. IR light is not
scattered as readily as visible light.
Another example was shown in class, a thermal
image
of
a
house. These are photograps of infrared light that
is being emitted (not reflected light) by a house. Remember that
the amount of energy emitted by an object depends strongly on
temperature (temperature to the 4th power in the Stefan-Boltzmann
law). Thus it is possible to see hot spots that emit a lot of
energy and appear "bright" and colds spots. Photographs like
these are often used to perform an "energy audit" on a home, i.e. to
find spots where energy is being lost. Once you locate one of
these hot spots you can add insulation and reduce the energy loss.
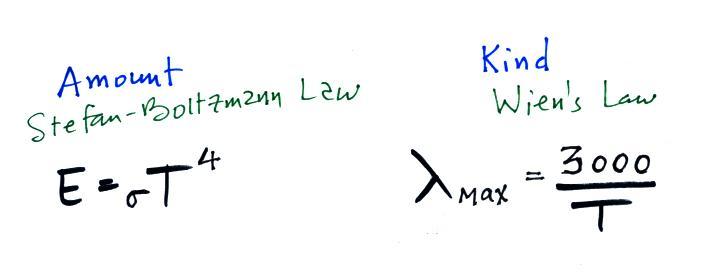
Here are the rules for the amount and kind (wavelength of peak
emission) of radiation emitted by an object.
Let's look at the light emitted by
the sun and the earth.
The curve on the left is for the sun. We have used Wien's
law and a temperature of 6000 K to calculate λmax
and got
0.5 micrometers. This is green light; the sun emits more green
light than any other kind of
light. The sun doesn't appear green because it is also emitting
lesser amounts of violet, blue, yellow, orange, and red - together this
mix of
colors appears white. 44% of the radiation emitted by the sun is
visible light, Very nearly half of sunlight (49%) is IR light
(37% near IR + 12% far IR). 7% of sunlight is ultraviolet
light. More than half of the light emitted by the
sun (the IR and UV light) is invisible.
100% of the light emitted by the earth (temperature = 300 K) is
invisible IR light. The
wavelength of peak emission for the earth is 10 micrometers.
Because the sun (surface of the
sun) is 20 times hotter than the earth the sun's
surface emits energy at a much higher rate than the earth. Note
the
vertical
scale
on
the
earth
curve
is
different
than
on
the
sun
graph. If both the earth and sun were plotted with the same
vertical scale, the earth curve would be too small to be seen.
In the demonstration in class last Friday we also learned that
ordinary tungsten bulbs (incandescent
bulbs) produce a lot of
wasted energy. This is because they emit a lot of invisible
infrared light that doesn't light up a room (it will warm up a room but
there are better ways of doing that). The light that they do
produce is a warm white color (tungsten bulbs emit lots of orange, red,
and yellow light and not much blue, green or violet).
Energy
efficient
compact
fluorescent
lamps
(CFLs)
are
being
touted
as
an
ecological
alternative
to
tungsten bulbs because
they use substantially less electricity, don't emit a lot of
wasted infrared light, and also last
longer. CFLs come with
different color temperature ratings.
The bulb with the hottest
temperature rating (5500 K ) in the figure
above is meant to mimic or simulate sunlight (daylight). The
temperature of
the sun is 6000 K and lambda max is 0.5 micrometers. The spectrum
of the 5500 K bulb is similar.
The tungsten bulb (3000 K) and the CFLs with temperature ratings
of
3500 K and 2700 K produce a warmer white.
Three CFLs with the temperature ratings above were set up in class
so
that you could see the difference between warm and cool white
light. Personally I find the 2700 K bulb "too warm," it makes a
room
seem gloomy and depressing (a student in class once said the
light resembles Tucson at night). The 5500 K bulb is "too cool"
and
creates
a stark sterile atmosphere like you might see in a
hospital corridor. I prefer the 3500 K bulb in the
middle.
The figure below is from an article
on compact fluorescent lamps in Wikipedia for those of you that weren't
in class and didn't see the bulb display. You can
see a clear difference between the cool white bulb on the left
in the figure below and the warm white light produced by a tungsten
bulb (2nd from the left) and 2 CFCs with low temperature ratings (the 2
bulbs at right).
There is one downside to these energy efficient CFLs. The
bulbs
shouldn't just be discarded in your ordinary household trash because
they contain mercury. They should be disposed of properly (at a
hazardous materials collection site or perhaps at the store where they
were purchased).
It probably won't be long before LED bulbs begin to
replace tungsten and CFL bulbs. At the present time the LED bulbs
are pretty expensive.
We now
have most of the tools we will need to begin to study energy balance on
the earth. It will be a balance between incoming sunlight
energy and outgoing energy emitted by the earth. This will
ultimately lead us to an explanation of the atmospheric greenhouse
effect.
We will first look at the simplest kind of situation, the earth without
an atmosphere (or at least
an atmosphere without greenhouse gases). The next figure is on p.
68 in the
photocopied Classnotes. Radiative equilibrium is really just
balance between incoming and outgoing radiant energy. Pages 68
and 69 were somehow left out of the photocopied ClassNotes.
Copies of these two pages were handed out in class.
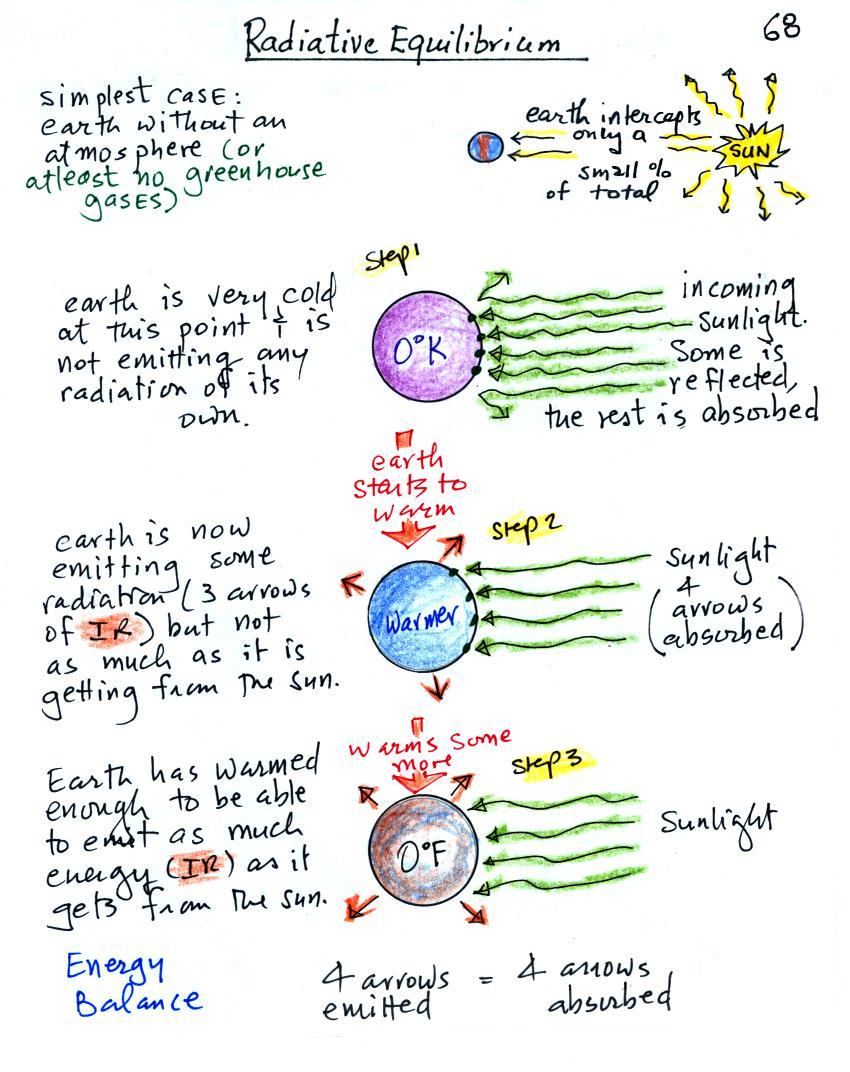
You might first wonder how it is possible for the earth (with a
temperature
of around 300 K) to be in energy
balance with the sun (6000 K). At the top right of the figure you
can see that because the earth is located about 90
million miles
from the sun and it only absorbs a very small fraction of the
total energy emitted by the sun.
To understand how energy balance occurs we start, in Step #1, by
imagining that the earth starts out very cold (0 K) and is
not emitting
any EM radiation at all. It is absorbing sunlight however (4
of
the
5
arrows
of
incoming
sunlight
in
the
first
picture
are absorbed, 1 of the arrows is being reflected) so
it
will
begin
to
warm
This is like opening a bank account, the balance
will be zero at first. But then you start making deposits and the
balance
starts to grow.
Once the earth starts to warm it will also begin to emit EM
radiation, though not as much as it is getting from the sun (the
slightly warmer earth in the middle picture is now colored blue).
Only the four arrows of incoming sunlight that are absorbed are shown
in the
middle figure. The two arrows of reflected sunlight have been
left off because they don't really play a role in energy balance (reflected
sunlight
is
like
a
check
that
bounces
-
it
really doesn't affect your bank account
balance). The earth is emitting 3 arrows of IR light
(in red). Because the earth is still gaining more
energy than it is losing the
earth will warm some more. Once you
find money in your bank account you start to spend it. But as
long as deposits are greater than the withdrawals the balance will grow.
Eventually it will warm enough that the earth (now shaded brown
& blue)
will
emit the same amount
of energy as it absorbs from
the sun. This is radiative equilibrium, energy balance (4 arrows
of absorbed energy are balanced by 4 arrows of emitted energy).
The
temperature at
which this occurs is about 0 F.
That is called the temperature of radiative equilibrium (it's about 0 F
for the earth).
Note that it is the amounts of energy not the kinds of energy that
are important. Emitted radiation may have a different wavelength
than
the absorbed energy. That doesn't matter. As long as the
amounts are
energy the earth will be in energy balance.
Before we
start to look at radiant energy balance on the earth with an atmosphere
we
need to learn about filters. The atmosphere will filter sunlight
as it
passes through the atmosphere toward the ground. The atmosphere
will
also filter IR radiation emitted by the earth as it trys to travel into
space.
We will first look at the effects simple blue, green, and red glass
filters have on visible light. This is just to be able
to interpret a filter absorption curve or graph.
If you try to
shine white light (a
mixture of all the colors) through a
blue filter, only the blue light passes through. The filter
absorption curve shows 100% absorption at all but a narrow range of
wavelengths that correspond to blue light. The location of the
slot or gap in the absorption curve shifts a little bit with the green
and red filters.
The following figure is a simplified, easier to
remember,
representation of the
filtering effect of
the atmosphere on UV, VIS, and IR light (found on p. 69 in the
photocopied notes). The figure was redrawn after class.
You can use your own eyes to tell
you what effect the
atmosphere has on visible light. Air is clear, it is
transparent. The atmosphere transmits visible light.
In our simplified representation oxygen and ozone make the
atmosphere pretty nearly completely opaque to UV light (opaque is the
opposite of transparent and means
that light is blocked or absorbed; light can't pass through an opaque
material). We
assume that the
atmosphere absorbs all incoming UV light, none of it makes it to the
ground. This is of course not entirely realistic.
Greenhouse gases make the
atmosphere a
selective absorber of IR light - the air absorbs certain IR wavelengths
and
transmits others. It is the atmosphere's ability to absorb
jcertain wavelengths of infrared light that produces the
greenhouse effect and warms the surface of the earth. I didn't
mention it in class but greenhouse gases also emit infrared
light. This is an important part of the greenhouse effect,
something we'll return to on Friday after the quiz.
Note "The atmospheric window"
centered at 10 micrometers. Light emitted by the earth
at this
wavelength (and remember 10 um is the wavelength of peak emission for
the earth) will pass through the atmosphere. Another transparent
region, another window, is found in the visible part of the spectrum.