The
top
two
equations
show
how ozone is produced in the stratosphere.
Ultraviolet (UV) light splits an O2
molecule into two O atoms
(photodissociation).
Each of the O atoms can react with unsplit O2 to make O3 (ozone).
Ozone is destroyed when it absorbs UV light and is split into O
and O2
(the two pieces move away from each other and don't recombine and
remake ozone). This is how the ozone protects
us. O3 is also
destroyed when it reacts with an oxygen
atom (thereby removing one of the "raw ingredients" used to make
ozone). Two molecules of ozone can also react with each other to
make 3 molecules
of O2
(probably the least likely of the three possibilities just because
there aren't many ozone molecules around to react with each other).
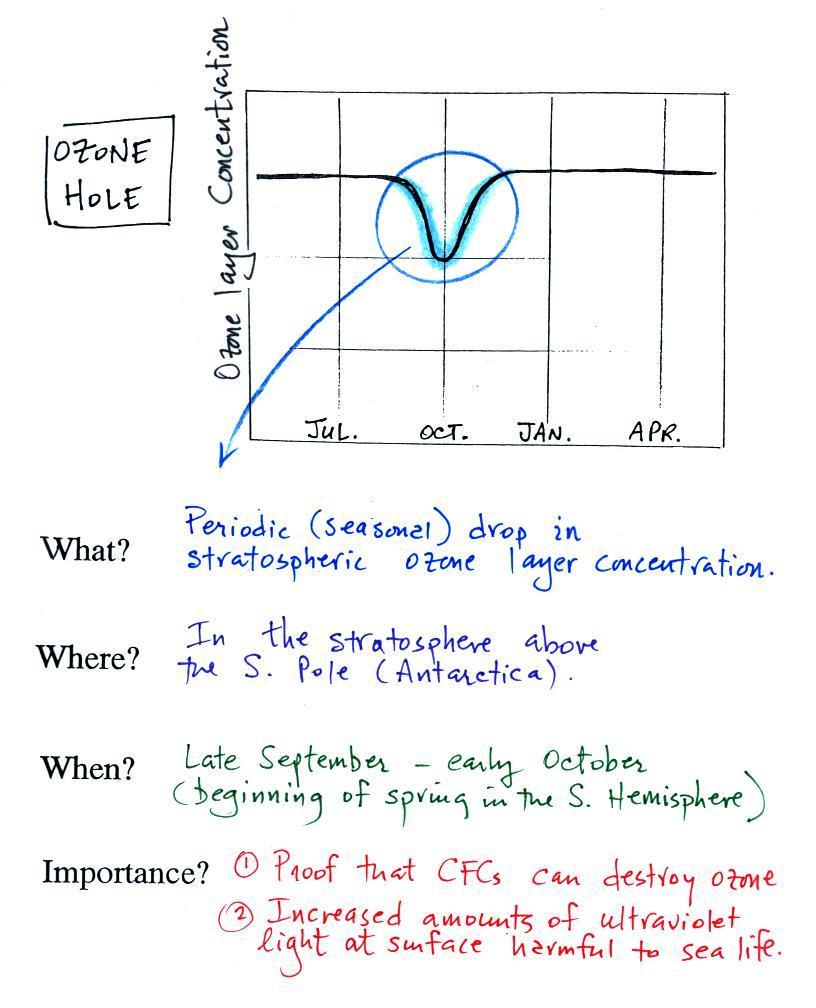
The ozone
concentration in the
stratosphere will shift up and down until the natural rates of
production and
destruction
balance each other (analogous to your bank account not changing once
the amounts of money being deposited and withdrawn are equal).
The black box represents the O3 layer concentration once
equilibrium is achieved. If an
additional man-caused destruction process is added (dotted red arrow)
the ozone layer concentration will decrease (sort of like someone else
coming along and starting to spend some of the money in your bank
account, your balance will decrease).
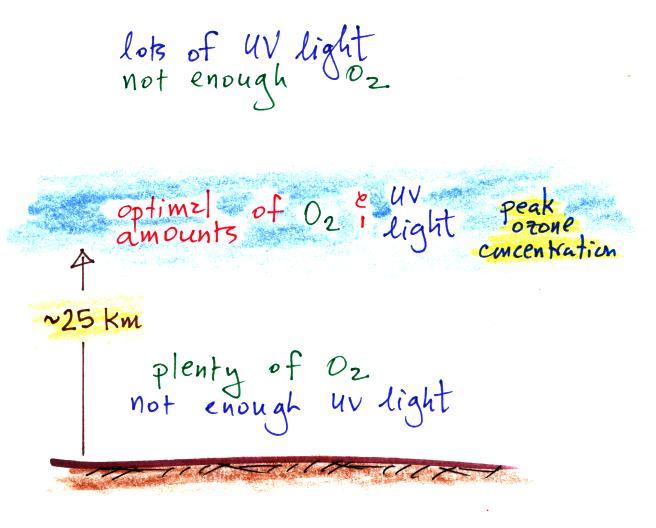
Knowing that you need O2
and UV light to make ozone,
you can
begin
to understand why the ozone layer is found in the middle of the
atmosphere.