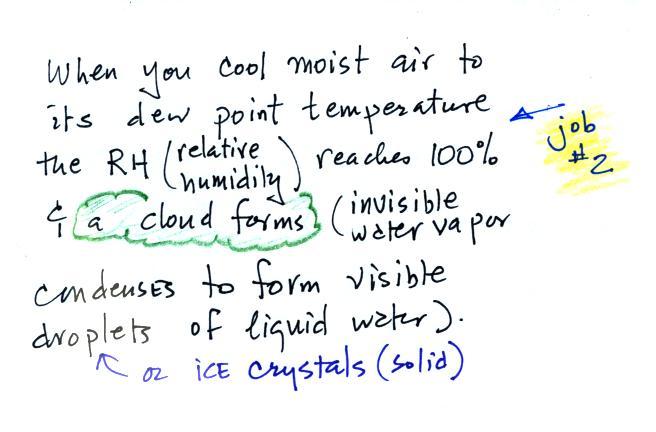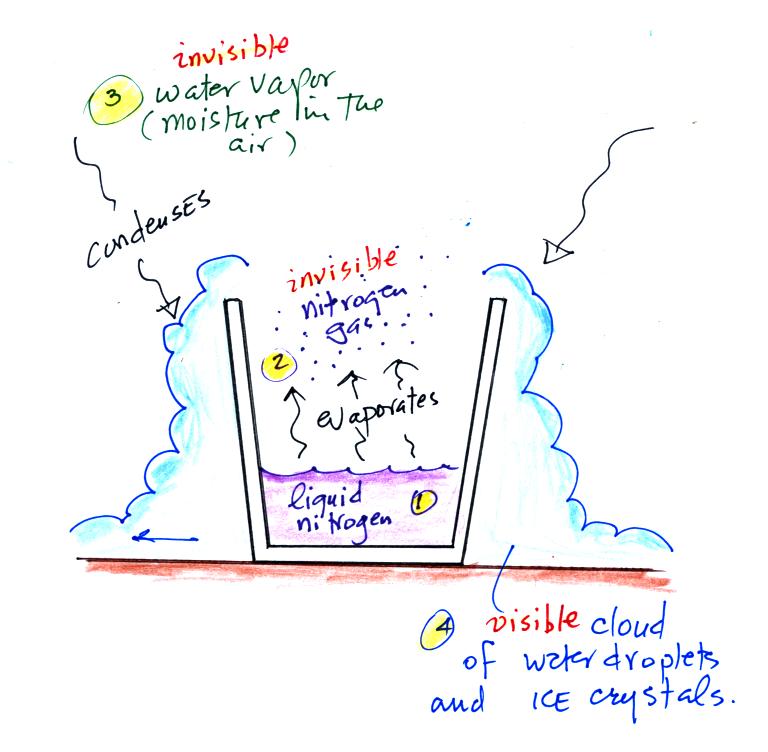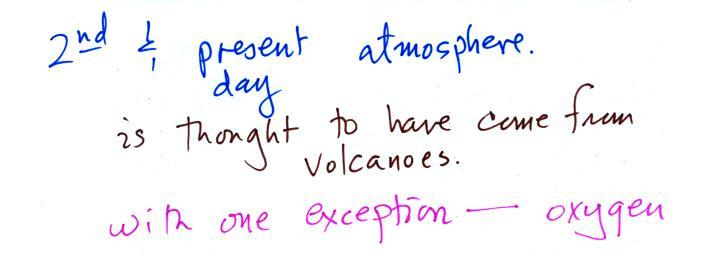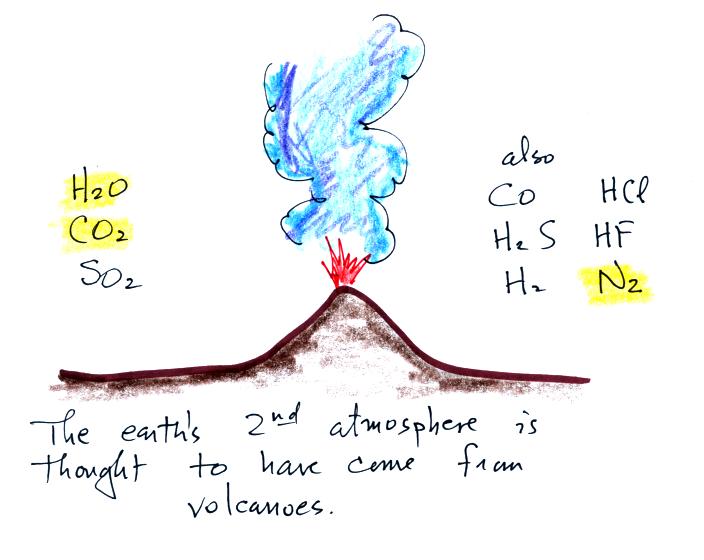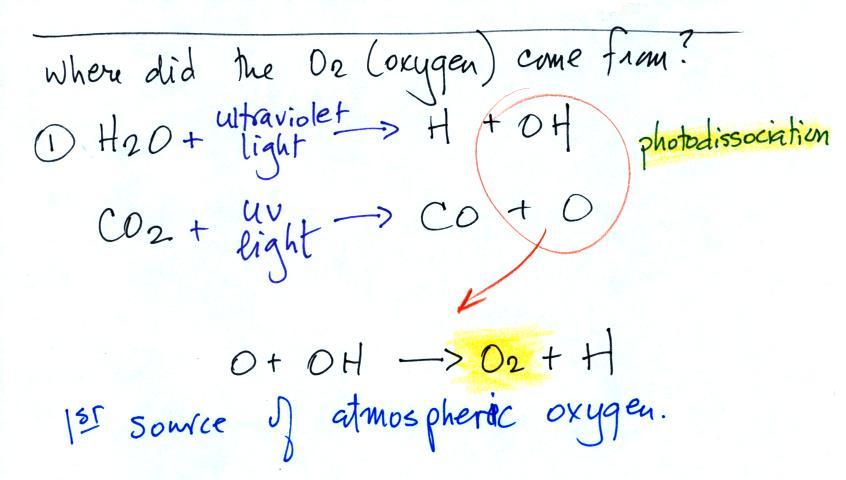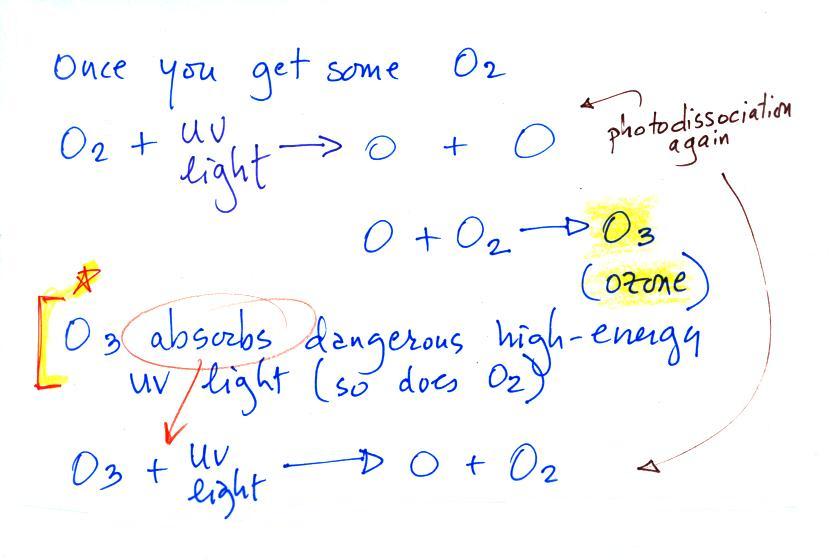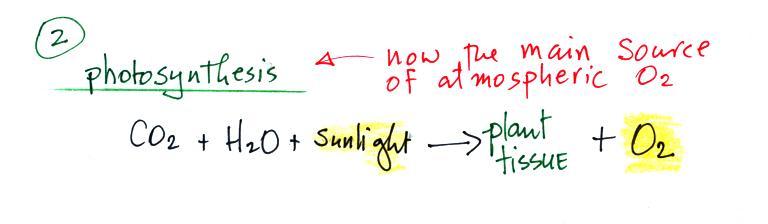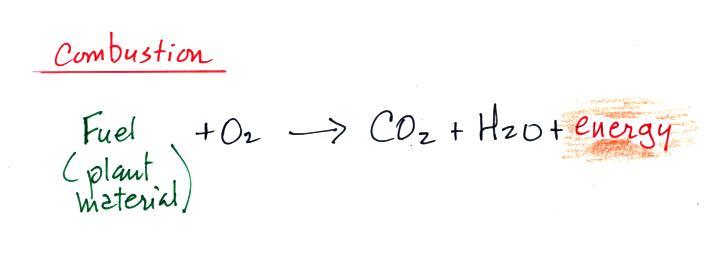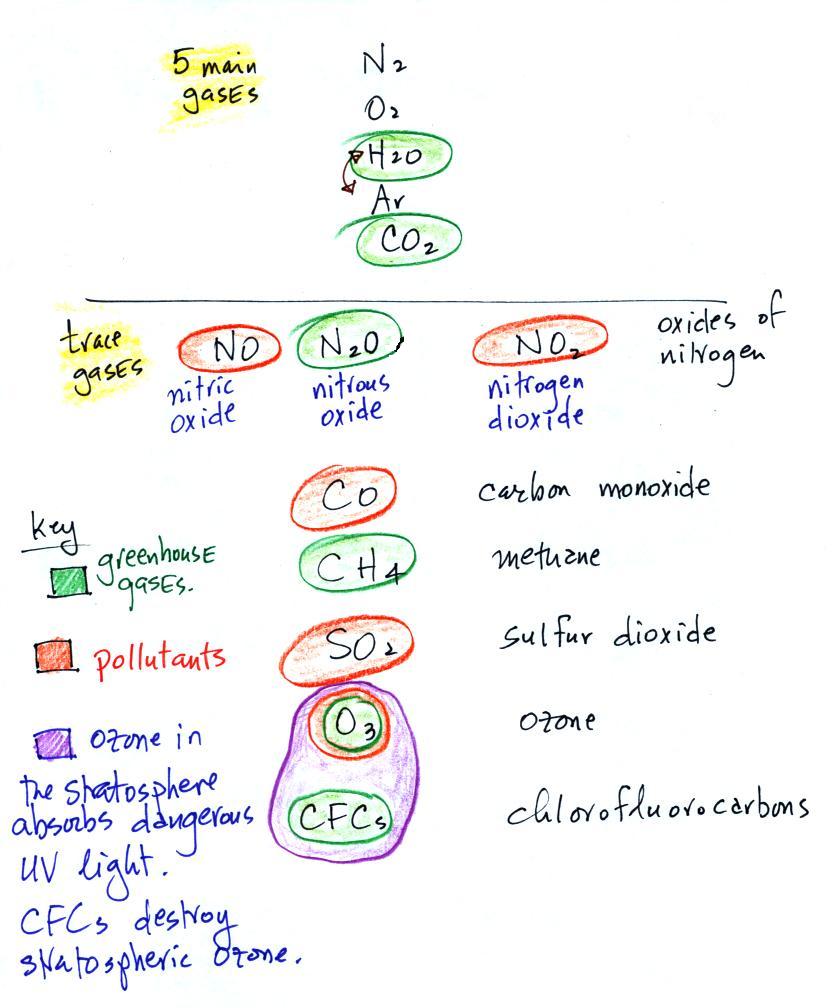
Carbon monoxide, nitric oxide, nitrogen dioxide, ozone, and
sulfur
dioxide are some of the major air pollutants. We'll cover 3 of
these in more detail next week.
Water vapor, carbon dioxide,
methane, nitrous oxide (N2O
=
laughing
gas),
chlorofluorocarbons,
and
ozone
are
all
greenhouse
gases.
Increasing atmospheric concentrations of these gases are responsible
for the current concern over climate change and global warming.
We'll
discuss this topic and learn more about how the
greenhouse effect actually works later in the course.
Ozone has sort of a Dr. Jeckyl and Mr. Hyde personality
(i) Ozone
in the
stratosphere (a layer of the atmosphere between about 10 and 50
km altitude) is beneficial because it absorbs dangerous high energy
ultraviolet
(UV) light coming from the sun. Without the protection of the
ozone layer, life as we know it would not exist on the surface of the
earth. It was only after ozone started to buildup in the
atmosphere that life could move from the oceans onto land.
Chlorofluorocarbons are of concern in the atmosphere
because they destroy stratospheric ozone.
(ii) In
the
troposphere (the bottom 10 kilometers or so of the atmosphere) ozone is
a
pollutant and is one of the main ingredients in photochemical smog.
(iii) Ozone is also a greenhouse gas.