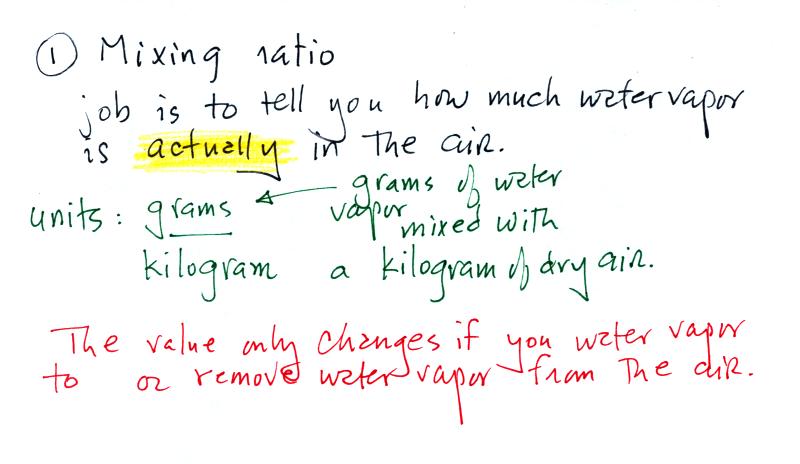
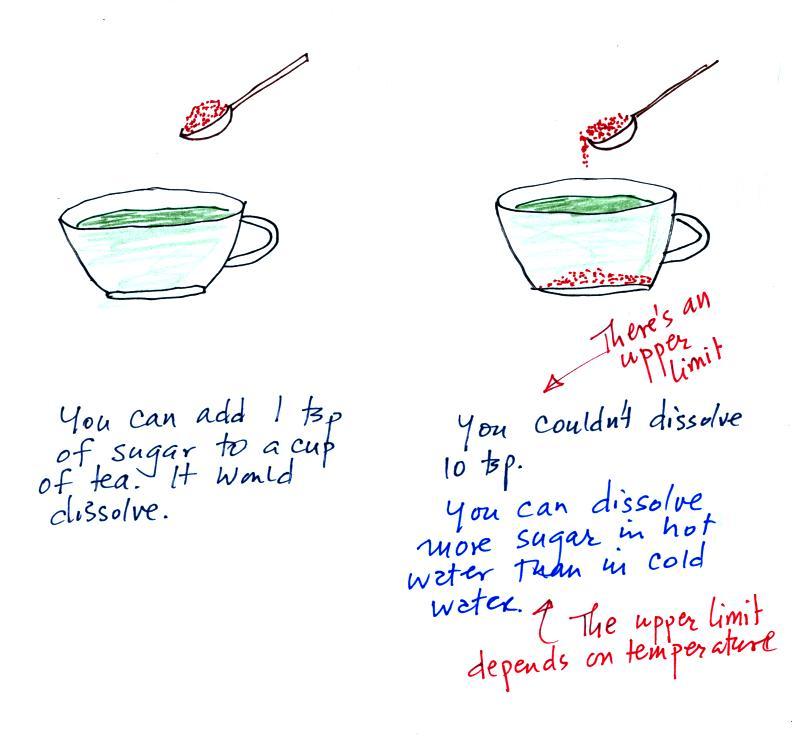
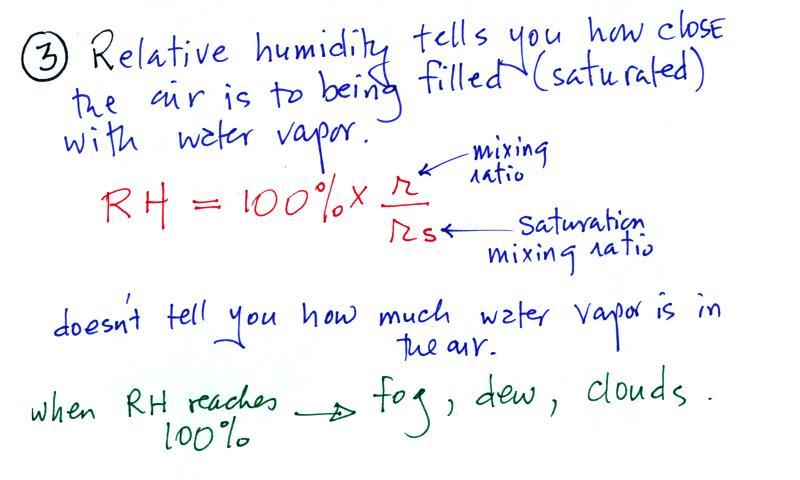
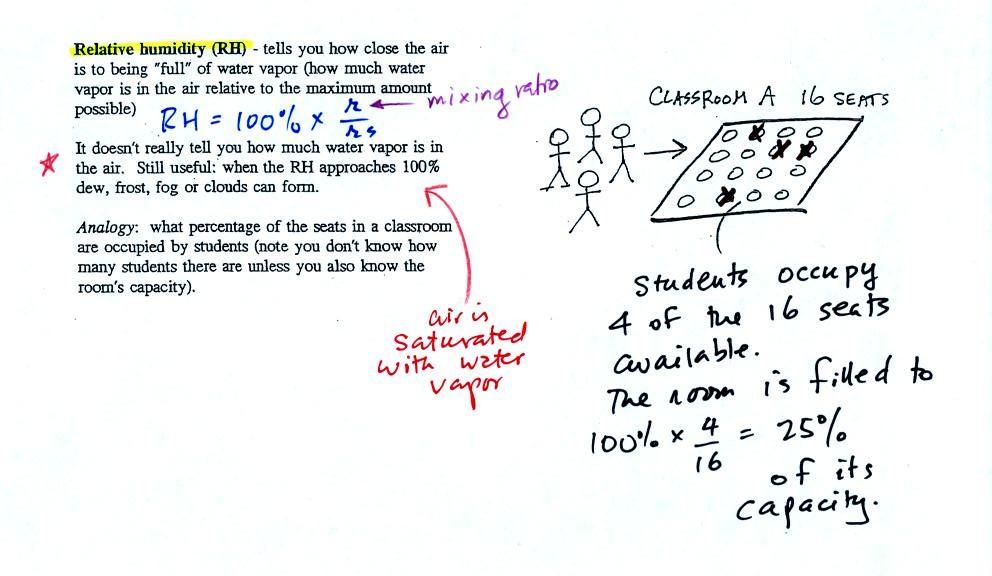
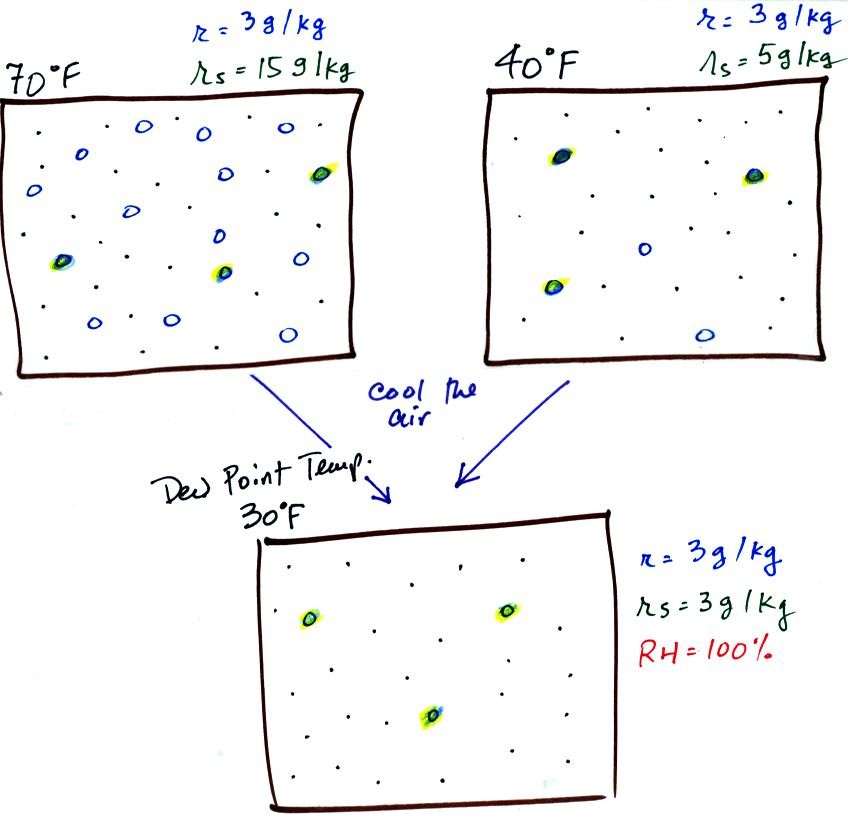
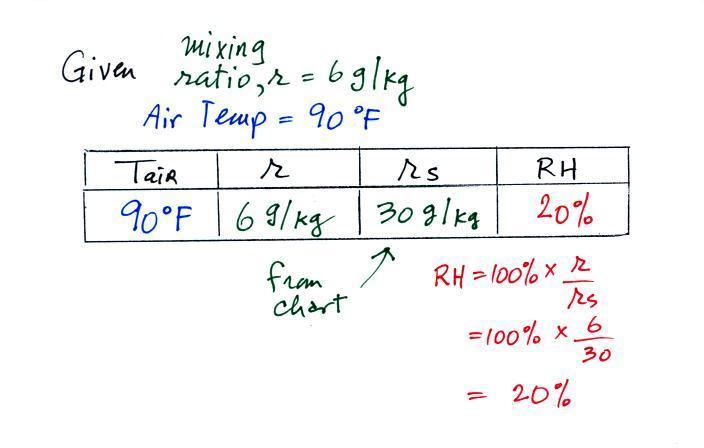
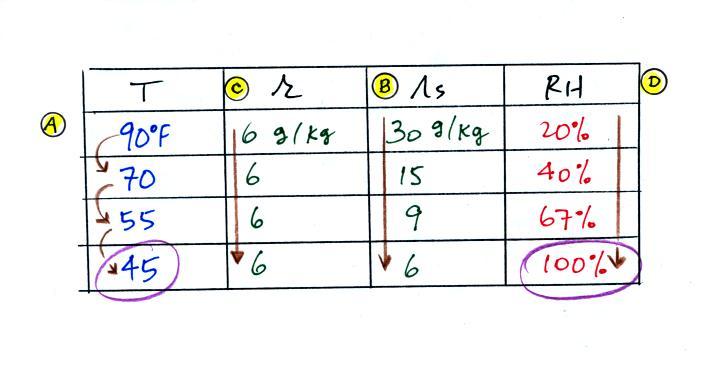
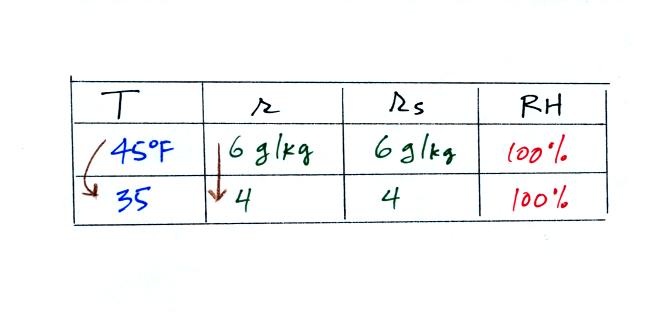
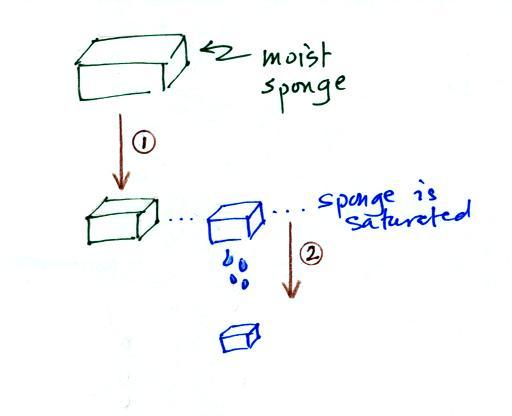
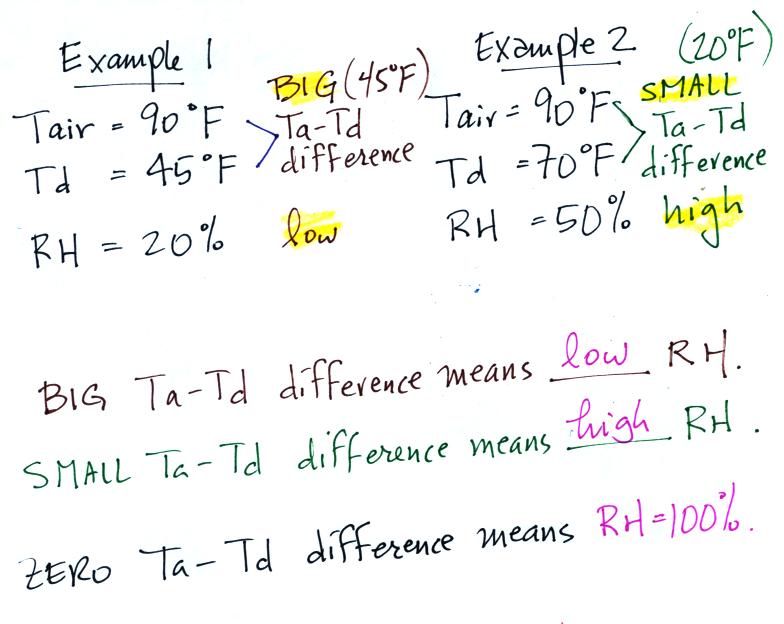
The sugar
dissolved in tea analogy is still helpful. Just as is the case
with water vapor in air, there's a limit to
how much sugar can be dissolved in a cup of hot
water. You could dissolve 1 tsp but I don't think you'd be able
to dissolve 10 tsp. You can dissolve more sugar in hot water
than in cold
water.
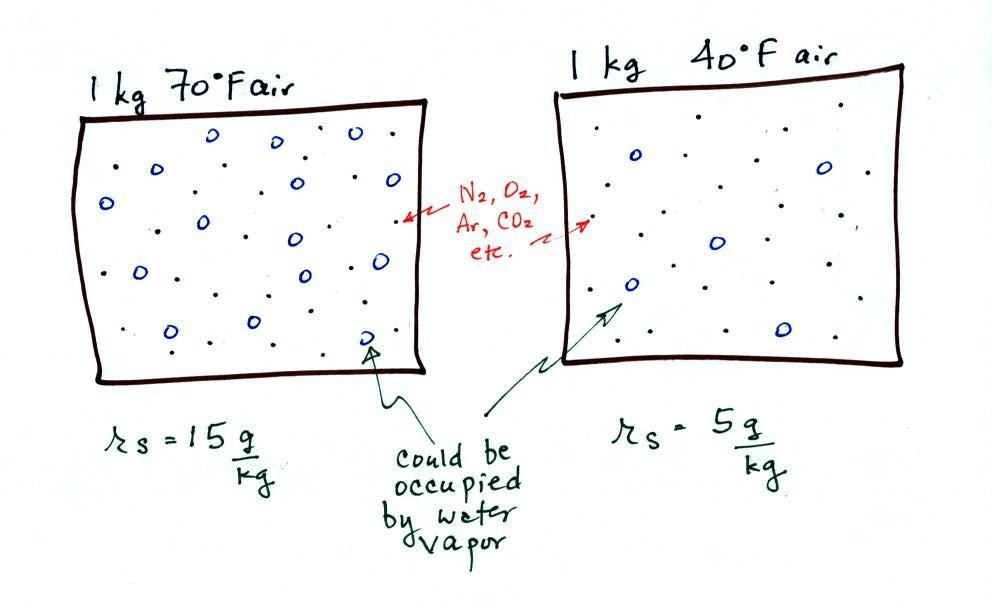
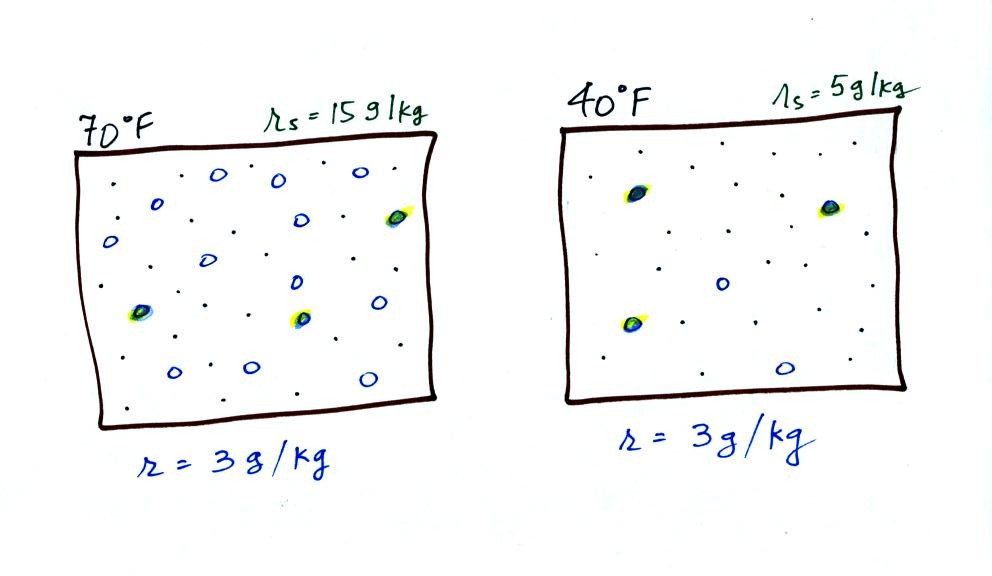
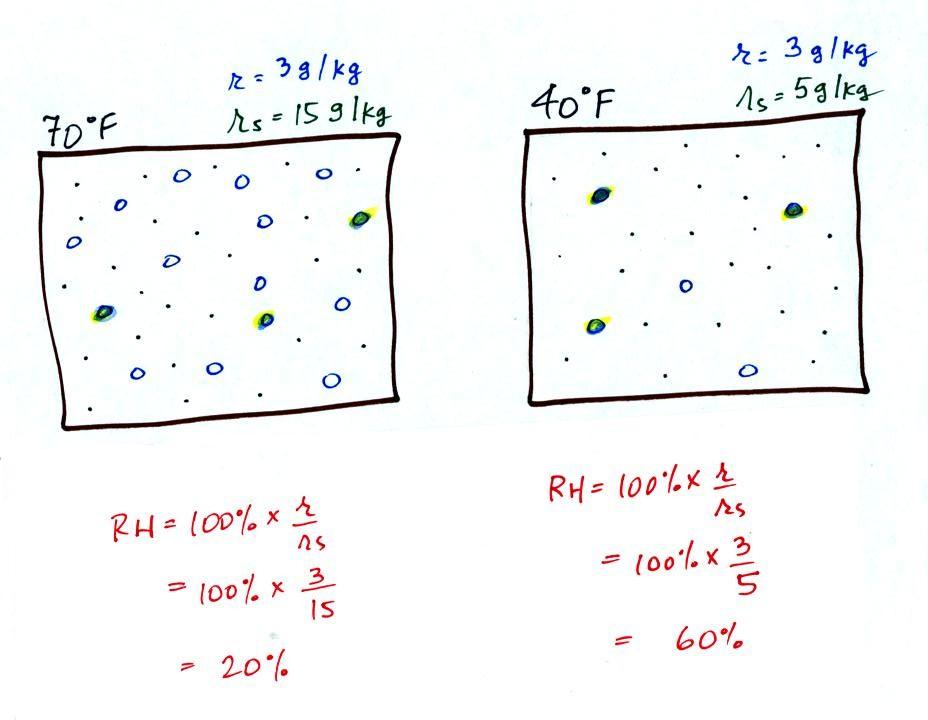
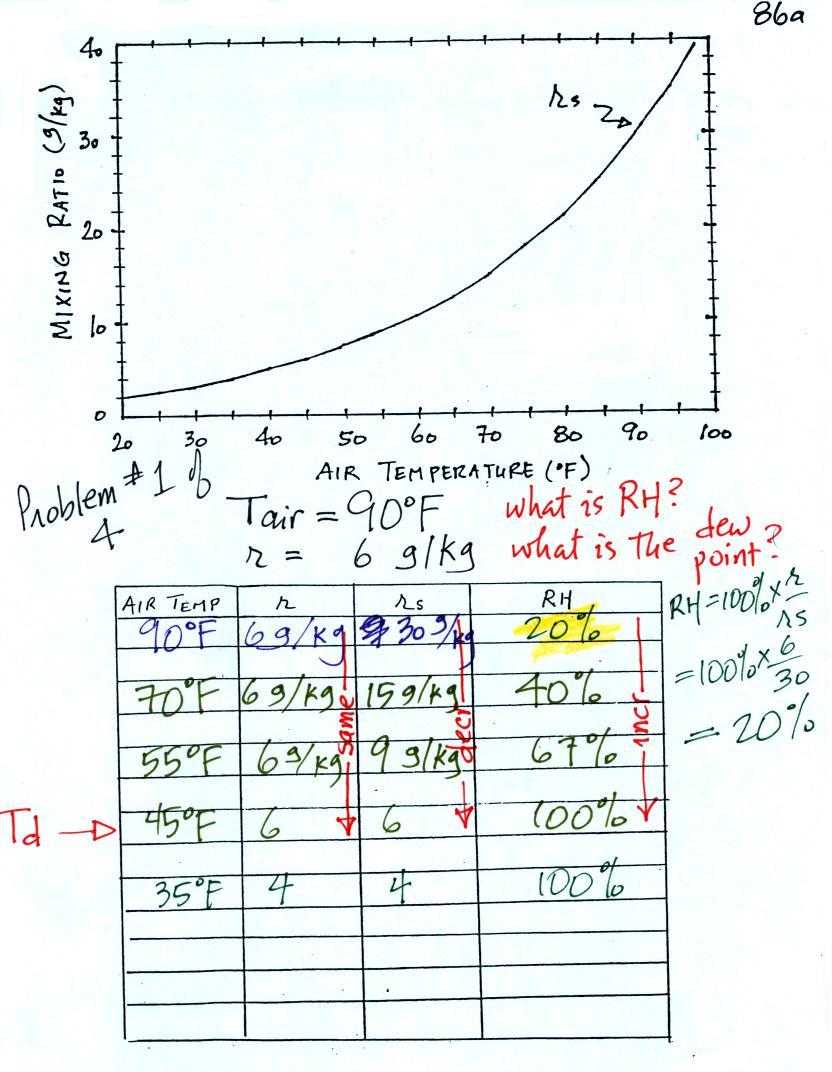
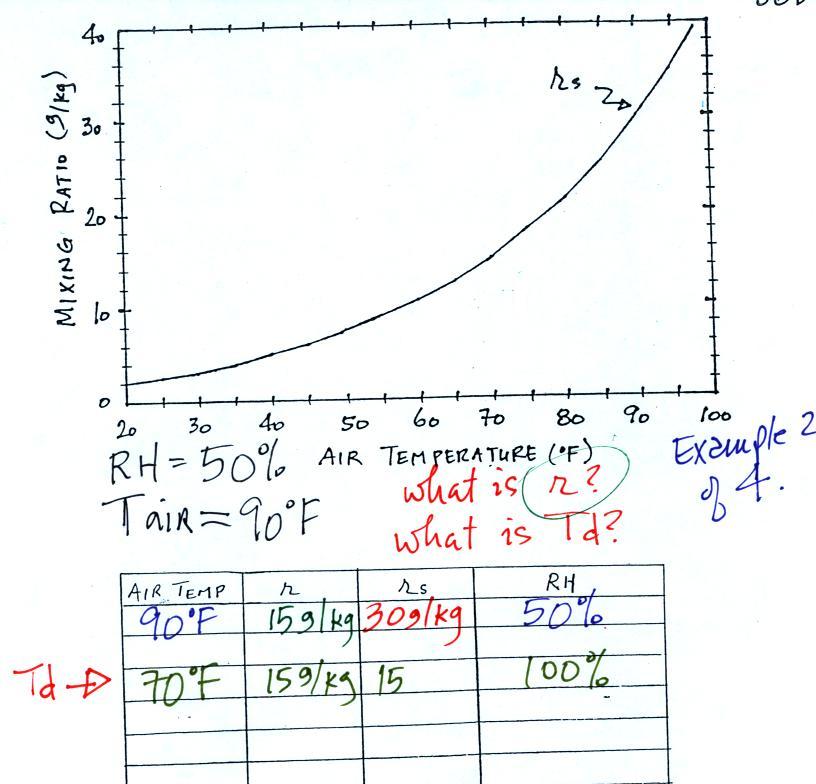
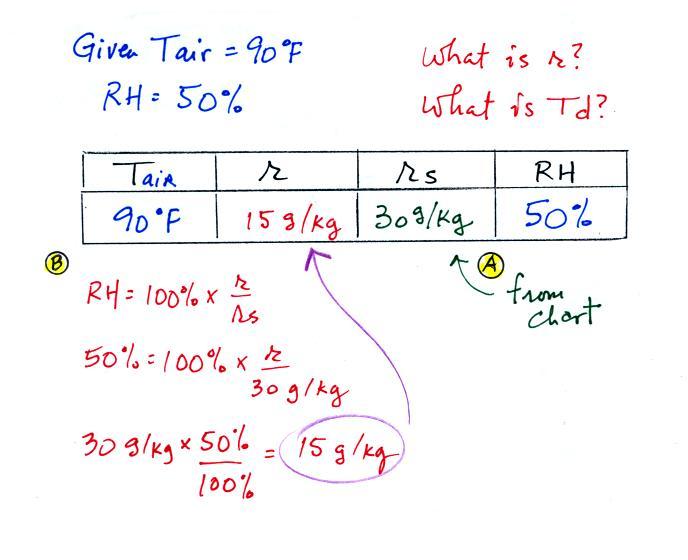
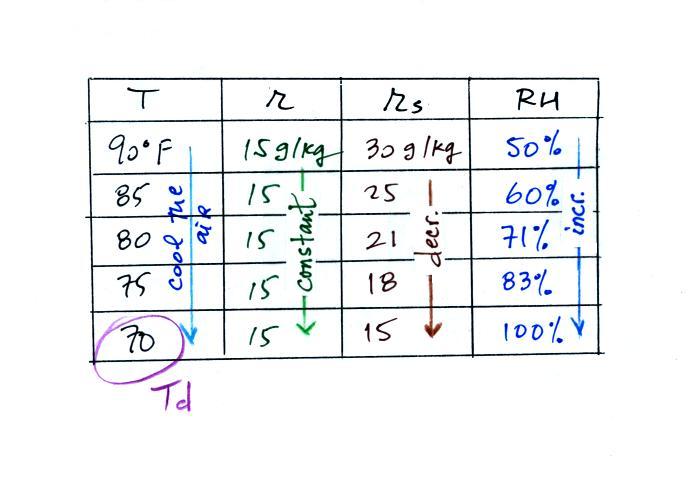
The dependence of saturation mixing ratio on air temperature is
illustrated below: