Tuesday Sep. 4, 2012
click here to
download today's notes in a more printer friendly format
Three songs from Crooked Still before class this morning ("Foggy Mountain
Special", "American
Tune", and "Baby,
What's
Wrong With You?")
The Practice Quiz is Thursday this week (during the second half of the
class period). The Practice Quiz Study Guide is now in its final
form. Reviews are scheduled for this afternoon and tomorrow
afternoon (the locations are on the Study Guide).
Now that we've learned something about
the composition of the atmosphere (and air pollutants) we will be
learning about some
of its other physical properties such as temperature, air
density, and air pressure. We'll also be interested in how they
change with altitude.

Two bottles, one containing mercury the
other an equal volume of water, were passed around class. Even
though the volumes were the same, the masses, weights, and densities
were very different.
Other books will define mass as
inertia or as resistance to change in motion (this comes from Newton's
2nd law of motion, we'll cover that later in the semester). The
next picture
illustrates both definitions.

A Cadillac and a volkswagen
have both stalled in an intersection. Both cars are made of
steel. The Cadillac is larger and has more steel, more stuff,
more mass. The Cadillac would be much harder to get moving than
the VW, it has
a larger inertia (it would also be harder to slow down and stop once it
is
moving).
Weight
is a force and depends on
both the mass of an object and the
strength of gravity. We tend to use
weight and mass
interchangeably
because we spend all our
lives on earth where gravity never changes. On the earth's
surface you determine the weight of an object by multiplying the
object's mass by g. As long as you're on the surface of the earth
g has a constant value; it's called the gravitational
acceleration.
The bottle of mercury that made its way through class (thanks for
returning the mercury) weighed more than the water. That was
something you could feel.
Here are a couple of questions that I asked in class.
We assume that all three objects are here on the earth.
To determine the weight you multiply the mass by the gravitational
acceleration. Since all three objects have the same mass and g is
a constant you get the same weight for each object. That's why we
use mass and weight interchangeably on the earth. Here was a
follow up question:
A student responded that an object would have
a different weight if were carried to the moon.
That's
correct.
Imagine carrying a brick from the earth to the
moon. It would be the same brick in both cases and would have the
same mass. The value of the gravitational acceleration on the
moon is
about 1/6th the value on the earth. So a brick that weighed 5
pounds on the earth would weigh less than 1 pound on the moon.
The brick would weigh almost 12 pounds on the surface on Jupiter.
Here's a
little
more
information (not
covered in class)
about
what
determines
the
value
of
the
gravitational
acceleration
(Newton's
Law
of
Universal Gravitation).
And before we move onto the next term here's a summary of mass and
weight units in both the metric and English (American) system.
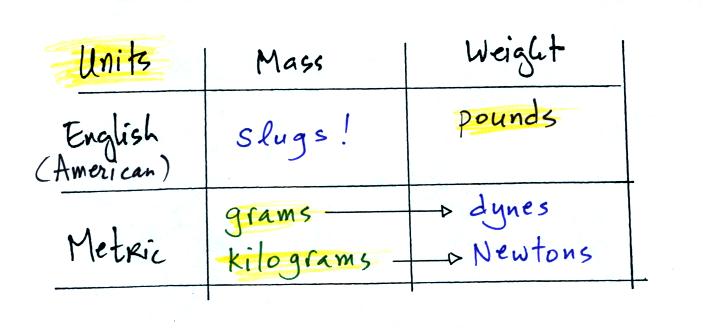
You've probably heard of pounds and
grams/kilograms. But, unless you've taken a physics class, you've
probably never run into dynes, Newtons, and slugs.
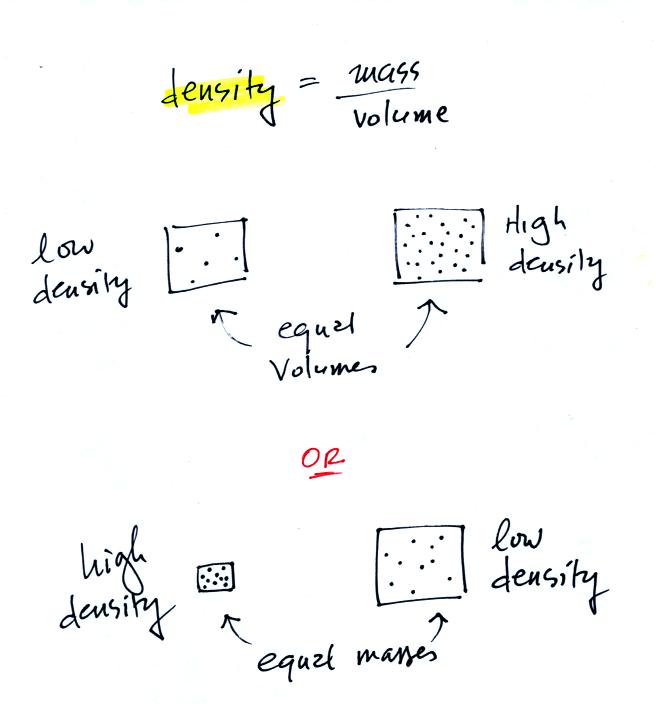
Density is the next term we need to look at.
In
the first example there is more mass (more dots, which symbolize air
molecules) in the right box than
in the left box. Since the two volumes are equal the box at right
has higher density. Equal masses are squeezed into different
volumes in the bottom example. The box with smaller volume has
higher density. Mercury
is more than 10 times more dense than water.
Now we're ready to define (and hopefully understand)
pressure.
It's a pretty important concept. A lot of what happens in the
atmosphere is caused by pressure differences. Pressure
differences cause wind. Large pressure difference (such as you
might find in a tornado or a hurricane) create powerful and destructive
storms.
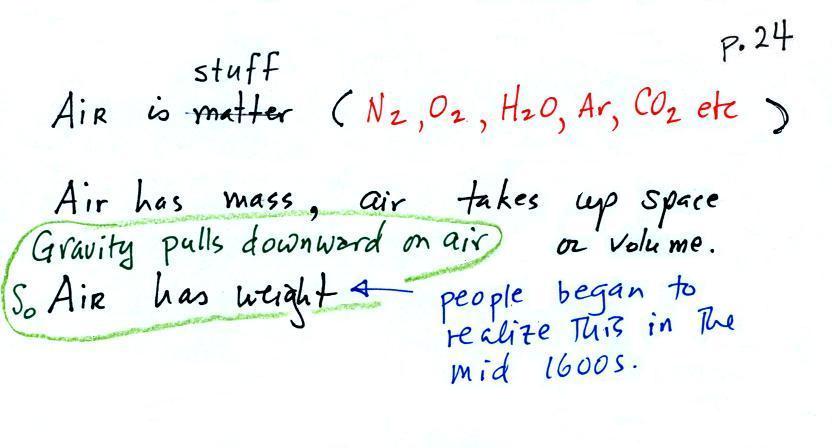
The air
that
surrounds the earth has mass. Gravity pulls downward on the
atmosphere giving it weight. Galileo conducted (in the 1600s) a
simple
experiment
to
prove
that
air
has
weight. The experiment
wasn't mentioned
in class.
Atmospheric pressure depends on, is
determined by, the weight of the air
overhead. This is one way, a sort of large scale representation,
of understanding air pressure.
Pressure is defined as force divided by area. In the case of
atmospheric pressure the weight of a column of air divided by the area
at the bottom of the column (as illustrated above).
Under normal conditions a 1 inch by 1 inch column of air
stretching
from sea level to the top of the atmosphere will weigh 14.7
pounds. Normal
atmospheric
pressure at sea level
is 14.7 pounds per square inch (psi, the units you use when you fill up
your
car
or
bike
tires
with
air).
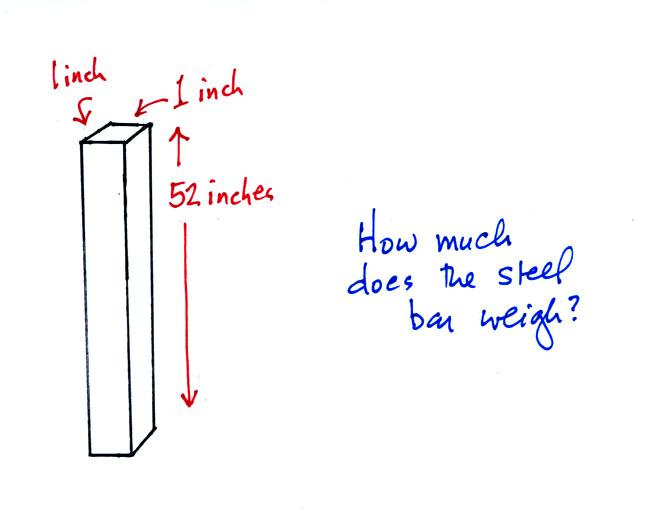
The iron bar sketched below was passed around class today.
You were supposed to estimate it's weight.
It also weighs 14.7 pounds. When you stand the bar on end,
the pressure at the bottom would be 14.7 psi.
So the weight of a 1" x 1" steel bar 52 inches long is the same
as a 1" x 1" column of air that extends from sea level to the top of
the atmosphere 100 or 200 miles (or more) high. The pressure at
the bottom of both would be 14.7 psi.
Psi are perfectly good pressure units, but they aren't the ones
that most meteorologists used.
Typical sea level
pressure is 14.7 psi or about 1000 millibars
(the
units used by meterologists and the units that we will use in this
class most of the time) or about 30 inches of mercury (refers to
the reading on a mercury barometer, we'll cover mercury barometers on
Friday, they're used to measure pressure). Milli means
1/1000 th. So 1000 millibars is the same as 1 bar. You
sometimes see typical sea level pressure written as 1 atmosphere.
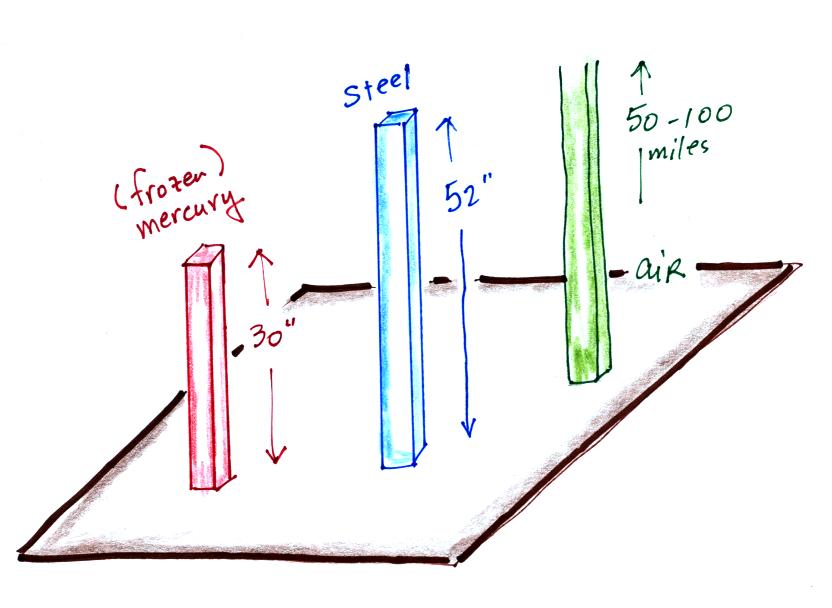
Mercury (13.6 grams/cm3)
is denser than steel ( about 7.9 grams/cm3
) so it would only take about a 30 inch tall column of mercury to
produce atmospheric presure.
Each of these columns would weigh 14.7 pounds.
The pressure at the base of each would be the same.
You never know whether something you learn in NATS 101 (or ATMO
170A1 as it's now called) will turn up. I lived and worked for a
short time in France (a very enjoyable and interesting period in my
life). Here's a picture of a car I owned when I was there (this
one is in mint condition, mine was in far worse
shape)
It's a Peugeot
404. After buying it I took it to the
service station to fill it with gas and to check the air pressure in
the tires. I was a little confused by the air
compressor though, the scale only ran from 0 to 3. I'm used to
putting 30 psi or so in my car tires (about 90 psi in my bike
tires). After staring at the scale for a while I finally realized
the numbers were pressures in "bars" not "psi". Since 14.7 psi is
equivalent to 1 bar, 30 psi would be about 2 bars. So I filled up
all the tires and carefully
drove off (one thing I quickly learned was you have to
watch out for in France is the "Priority to
the right" rule).
You
can
learn a
lot about pressure from bricks.
For example the photo below (taken in my messy office) shows two of the
bricks from class. One is sitting flat, the other is sitting on
its
end. Each brick weighs about 5 pounds. Would
the
pressure
at
the
base
of
each
brick
be
the
same
or
different
in this kind of
situation?
Pressure is determined by (depends on) weight so you might think the
pressures would be equal. But pressure is weight
divided by area. In this case the weights are the same but the
areas are different. In the situation at left the 5 pounds must
be divided by an area of about 4 inches by 8 inches = 32 inches.
That works out to be about 0.15 psi. In the other case the 5
pounds should be divided by a smaller area, 4 inches by 2 inches = 8
inches. That's a pressure of 0.6 psi, 4 times higher.
Notice also these pressures are much less the 14.7 psi sea level
atmospheric pressure.
The main reason I brought the bricks was so that you could understand
what happens to pressure with increasing altitude. Here's a
drawing of the 5 bricks stacked on top of each other.

The atmosphere is really no
different. Pressure at any level is
determined by
the weight of the air still overhead. Pressure decreases with
increasing altitude because there is less and less air remaining
overhead.
At sea
level altitude, at Point 1,
the pressure is normally about 1000 mb. That is determined by the
weight of all (100%) of the air in the atmosphere.
Some parts of Tucson, at Point 2, are 3000
feet above sea level (most
of central Tucson is a little lower than that around 2500 feet).
At
3000 ft. about 10%
of the
air is
below, 90% is still overhead. It is the weight of the 90% that is
still above that determines the atmospheric pressure in Tucson.
If 100% of the atmosphere produces a pressure of 1000 mb, then 90% will
produce a pressure of 900 mb.
Pressure is typically about 700 mb at the
summit of Mt. Lemmon (9000
ft. altitude at Point 3) and
70% of the atmosphere is overhead..
Pressure decreases rapidly with increasing
altitude. We will find that pressure changes more slowly if you
move horizontally. Pressure changes about 1 mb for every 10
meters of elevation change. Pressure changes much more slowly
normally if you move horizontally: about 1 mb in 100 km. Still
the small horizontal changes are what
cause the
wind to blow and what cause storms to form.
Point 4 shows
a
submarine
at
a
depth
of
about
30
ft.
or
so.
The
pressure
there
is
determined
by
the
weight
of
the
air
and
the
weight
of
the
water
overhead. Water is much denser and much heavier than
air. At 30 ft., the pressure is already twice what it would be at
the surface of the ocean (2000 mb instead of 1000 mb).
This
next figure explains the rate of pressure change as
you move or down in
the atmosphere depends on air density. In particular air pressure
will decrease more quickly when you move upward through high density
air than if you move upward through low density air.

There's a lot going on in this picture, we'll examine it step by
step.
1.The sea level pressure is the same, 1000 mb, in both pictures.
Since pressure is determined by the weight of the air overhead, the
weight of the air overhead in the left picture is the same as in the
right picture. The amount (mass) of air above sea level in both
pictures is the same.
2. There is a 100 mb drop in pressure in both air
layers. Pressure has decreased because air that was overhead (the
air between the ground the level of the dotted line) is now
underneath. Because the pressure change is the same in both
pictures the weight of the air layers are the same. The thin
layer at left has the same weight as the thicker layer at right.
Both layers contain the same amount (mass) of air.
3. Both layers contain the same amount (mass) of air.
The air in the layer at left is thinner. The air is squeezed into
a smaller volume. The air in the layer at left is denser than the
air in the layer at right.
4. To determine the rate of pressure decrease
you divide the pressure change (100 mb for both layers) by the distance
over which that change occurs. The 100 mb change takes place in a
shorter distance in the layer at left than in the layer at right.
The left layer has the highest rate of pressure decrease with
increasing altitude.
So both the most rapid rate of pressure decrease with altitude and
the densest air are found in Layer A.
The fact that the rate of pressure decrease with increasing
altitude depends on air density is a fairly subtle but important
concept. This concept
will come up 2 or 3 more times later in the semester. For
example, we will need this concept to explain why hurricanes can
intensify and
get as
strong as they do.