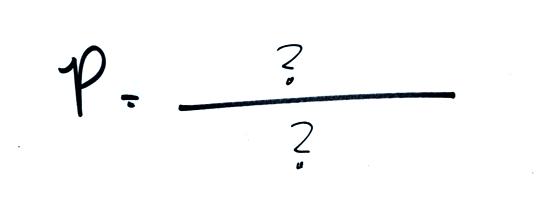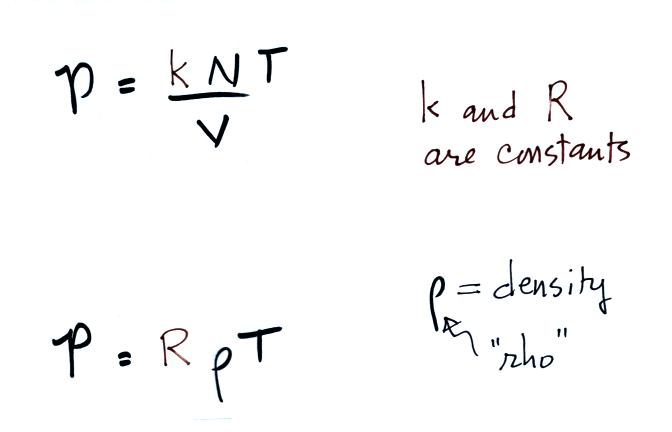We weren't done
yet. We had a little time to get started on a new topic, the
ideal gas
law. This is a first step in really
understanding why warm air rises and cold air sinks.

Hot air balloons rise (they also
sink), so does the relatively
warm air in a thunderstorm updraft (it's warmer than the air around
it). Conversely cold air sinks. The surface winds
caused by a thunderstorm downdraft (as shown above) can reach speeds of
100 MPH (stronger than many tornadoes) and are a serious weather hazard.
A full understanding of these rising and sinking
motions is
a
3-step process (the following is
from the bottom part of p. 49 in the photocopied ClassNotes). We
only had time to look at the first step today.

We will first learn about the ideal
gas law. That is an equation that tells you which properties of
the air
inside a
balloon work to determine the air's pressure. Then we will look
at Charles' Law, a special situation involving the ideal gas law (air
temperature and density change together in a way that keeps the
pressure
inside a balloon constant).
Then we'll learn about the
vertical forces that act on air (an upward
and a downward force).
Students working on Experiment #1 will need to understand the
ideal gas law to be able to fully explain why/how their experiment
works.
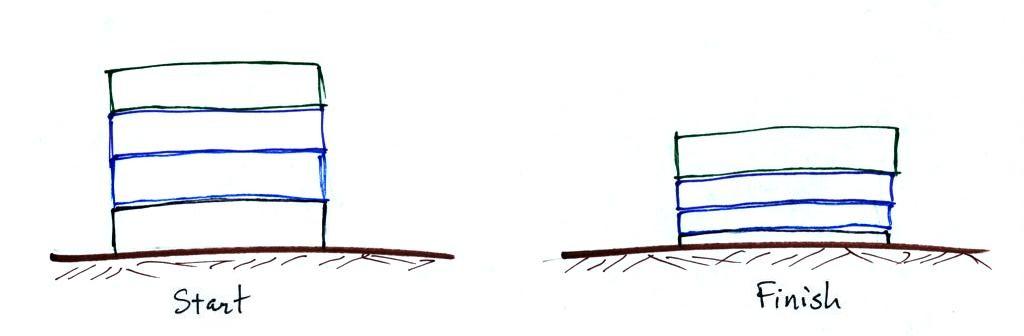
The figure above makes an important point: the air molecules in a
balloon "filled with air" really take up very little space. A
balloon filled with air is mostly empty space. It is the
collisions of air molecules traveling at 100s of miles per hour with
the inside walls of the balloon
that keep it inflated.
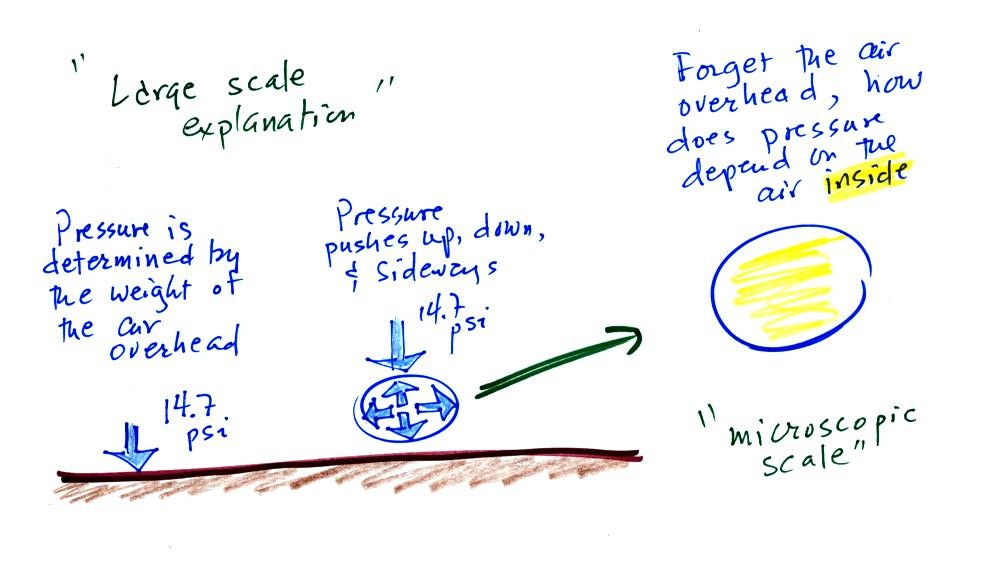
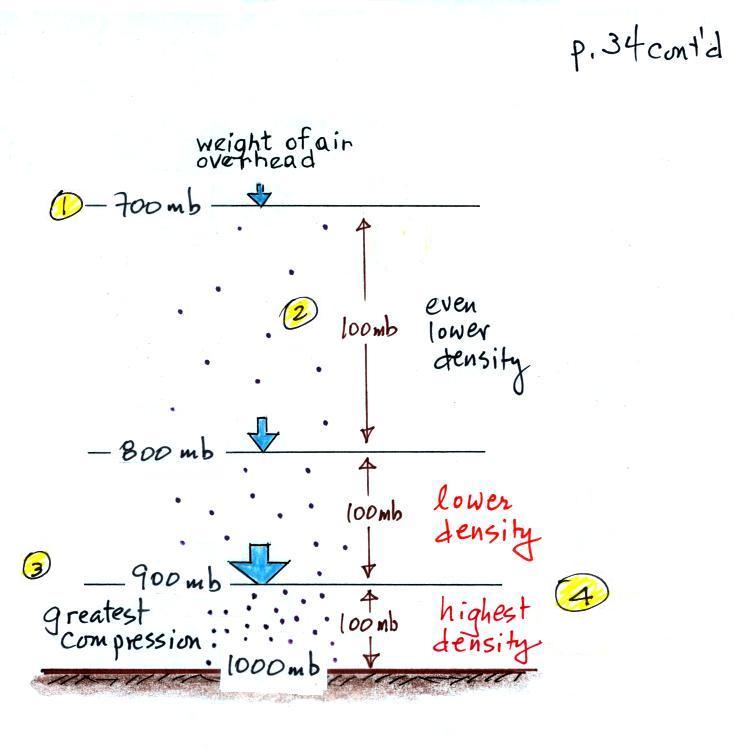
Up to this
point in the semester we
have been thinking of pressure as
being determined
by the weight of the air overhead. Air pressure pushes down
against the ground at sea level with 14.7 pounds of force per square
inch. If you imagine the weight of the atmosphere pushing down on
a balloon sitting on the ground you realize that the air in the balloon
pushes back with the same force. Air everywhere in the atmosphere
pushes upwards, downwards, and sideways.
The ideal gas law
equation is another way of thinking about air pressure, sort of a
microscopic scale version. We ignore
the atmosphere and concentrate on just the air inside the
balloon. We'll end up with an equation. Pressure (P)
will be on the left hand side of the equation. Relevant
properties of the air
inside the
balloon will be found on the right hand side.
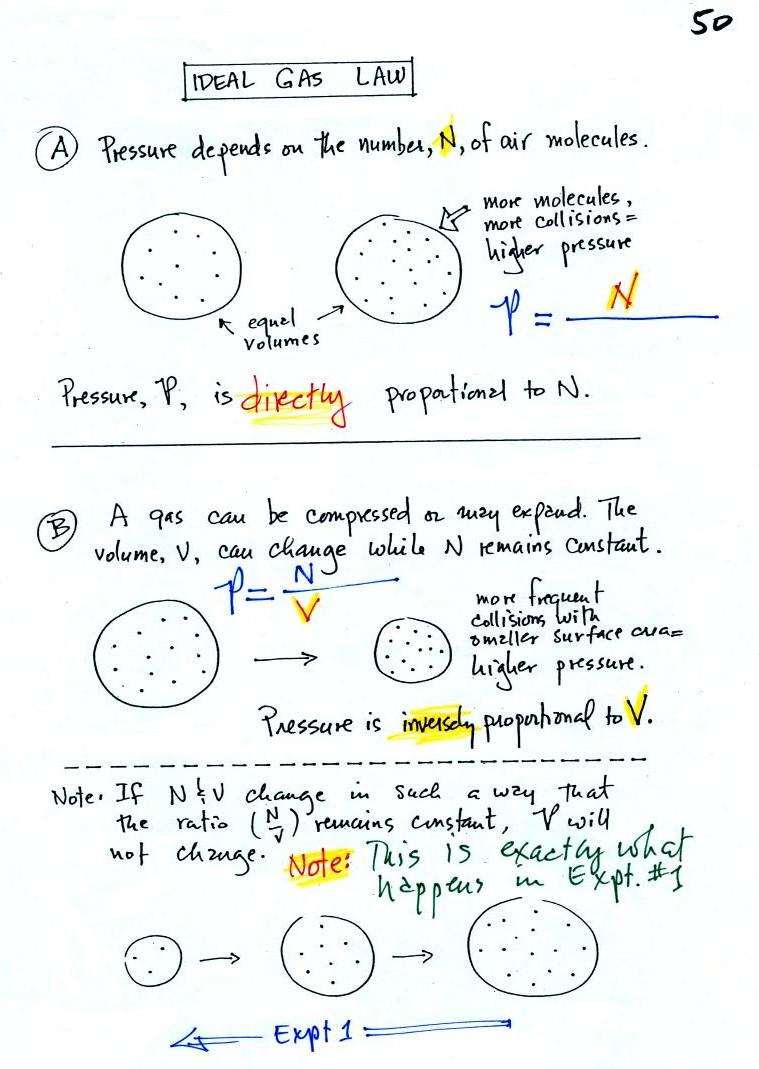
In A
the pressure produced by
the air
molecules inside a balloon will
first depend on how many air molecules are there, N. If there
weren't any air molecules at all there wouldn't be any
pressure. As you add more and more add to something like a
bicycle tire, the
pressure increases. Pressure is directly proportional to N; an
increase in N causes an increase in P. If N doubles, P also
doubles (as long as the other variables in the equation don't change).
In B
air pressure inside a balloon
also
depends on the size of the
balloon. Pressure is inversely proportional to volume, V
. If V were to double, P would drop to 1/2 its original value.
Note
it
is possible to keep pressure constant by changing N and V
together in just the right kind of way. This is what happens in
Experiment #1 that some students are working on. Here's a little
more detailed look at that experiment.
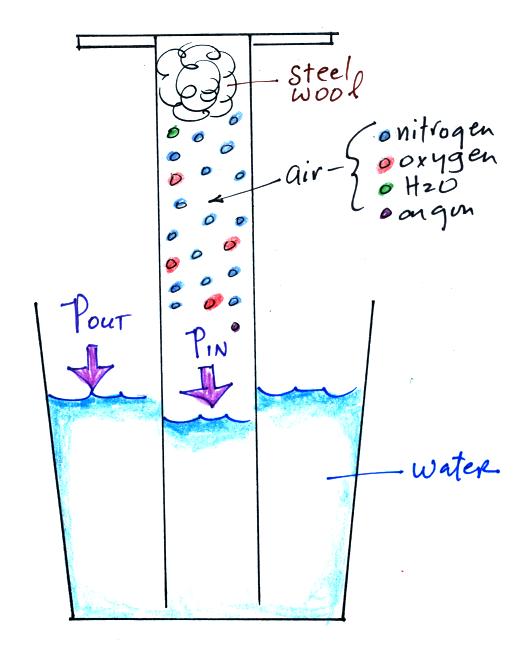
An air sample is trapped together with some steel wool inside a
graduated cylinder. The cylinder is turned upside down and the
open end is stuck into a glass of water. This is shown at left
above. Water will move into or out of the cylinder to keep Pout
equal to Pin. We assume
that neither
of
these pressures changes during the experiment. In this
experiment pressure is constant.
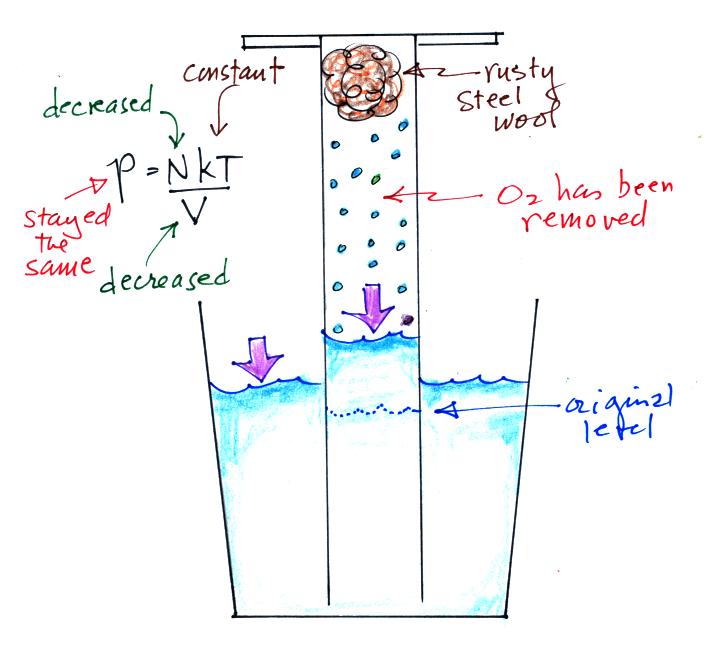
Oxygen in the cylinder reacts with steel wool to form rust.
Oxygen is
removed from the air sample which causes N (the total number of air
molecules) to decrease. Removal of oxygen would ordinarily cause
a drop in Pin.
But,
as
oxygen
is
removed,
water
rises
up
into
the
cylinder decreasing the air sample
volume. N and V both decrease in the same relative amounts and
the air sample pressure remains constant.
If you were to remove 20% of the air molecules, V would decrease
to 20% of its original value and pressure would stay constant. It
is the change in V that you can measure and use to determine the oxygen
percentage concentration in air.
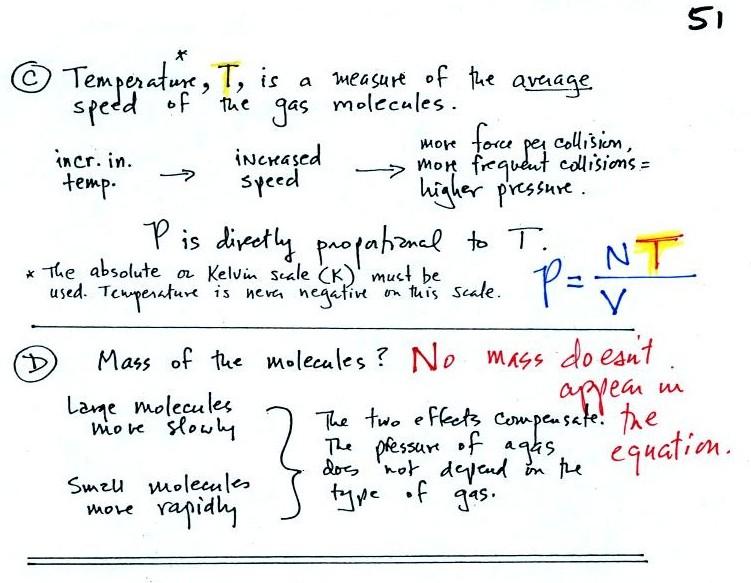
Part C: Increasing
the temperature of the gas in a balloon will cause the gas molecules to
move more quickly. They'll collide with the walls of the balloon
more frequently and rebound with greater force. Both will
increase the pressure. You shouldn't throw a can of spray paint
into a fire because the temperature will cause the pressure inside the
can to increase and the can could explode.
Surprisingly, as explained in Part
D,
the pressure
does
not depend on the mass of the
molecules. Pressure doesn't depend on the composition of the
gas. Gas molecules with a lot of mass will move slowly, the less
massive molecules will move more quickly. They both will collide
with the walls of the container with the same force.
The figure below (which replaces the bottom of p. 51 in the
photocopied
ClassNotes) shows two forms of the ideal gas law. The top
equation is the one we just derived and the bottom is a second slightly
different version. You can
ignore the
constants k and R if you are just trying to understand how a change in
one of the variables would affect the pressure. You only need the
constants when you are doing a calculation involving numbers (which we
won't be doing).
We'll come back to this topic and
make use of these equation in class on Thursday. I also mentioned
that if someone reminds me, I'll put both equations on the board for
you to use during the next quiz.