| Tair = 90 F |
r = ? |
| RH = ? |
Td = 50 F |
One of the dew point's jobs is the same as the mixing ratio - it gives you an idea of the actual amount of water vapor in the air. This problem will show that if you know the dew point, you can quickly figure out the mixing ratio and vice versa. Knowing the dew point is equivalent to knowing the mixing ratio.
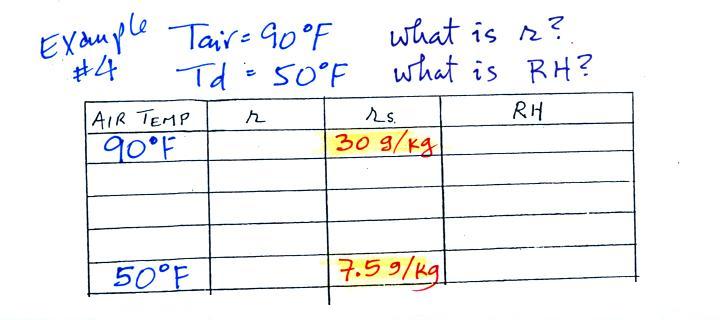
We enter the two temperatures given on a chart and look up the saturation mixing ratio for each.
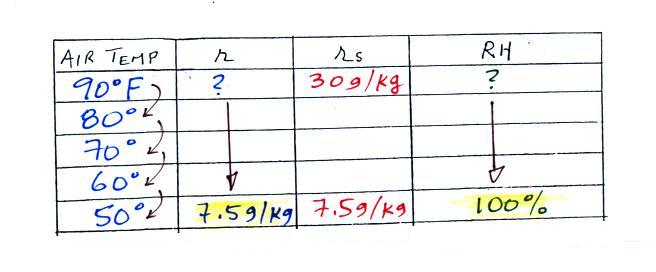
We ignore the fact that we don't know the mixing ratio. We do know that if we cool the 90 F air to 50 F the RH will become 100%. So on the 50 F row, we can set the mixing ratio equal to the value of the saturation mixing ratio at 50 F, 7.5 g/kg. The two have to be equal in order for the RH to be 100%.
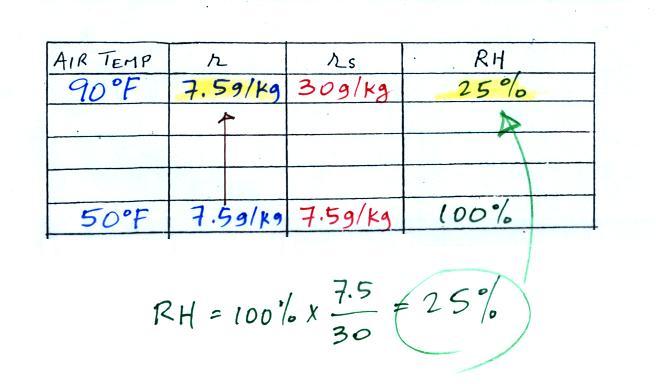
Remember back to the three earlier examples. When we cooled air to the the dew point, the mixing ratio didn't change. So the mixing ratio must have been 7.5 all along. Once we know the mixing ratio in the 90 F air it is a simple matter to calculate the relative humidity, 25%.
Drying moist air
The figure below is on p. 87 in the photocopied ClassNotes. It explains how you can dry moist air.
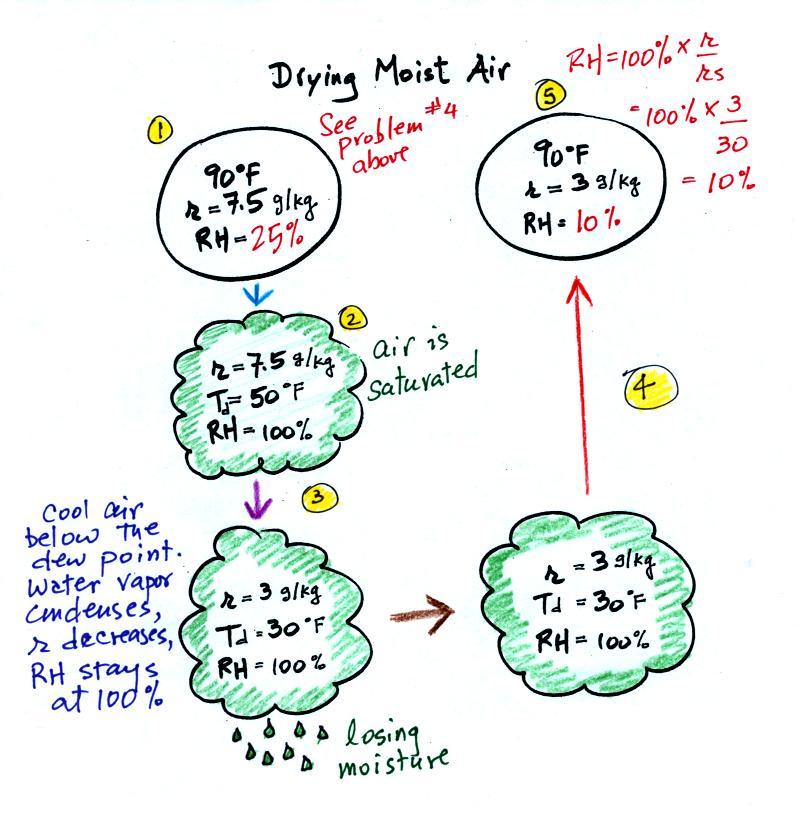
At Point 1 we start with some 90 F air with a relative humidity of 25%, fairly dry air. These are the same numbers that we had in Example Problem #4. We imagine cooling this air to the dew point temperature, 50 F. While doing that the mixing ratio, r, would stay constant. Relative humidity would increase and eventually reach 100%. A cloud would form (Pt. 2 in the figure above).
Then we continue to cool the air below the dew point, to 30 F. Air that is cooled below the dew point finds itself with more water vapor than it can contain. The excess moisture must condense (we will assume it falls out of the air as rain or snow). Mixing ratio will decrease, the relative humidity will remain 100%. When air reaches 30 F it contains 3 g/kg, less than half the moisture that it originally did (7.5 g/kg).
The air is being warmed back up to 90 F along Path 4. As it warms the mixing ratio remains constant. Cooling moist air raises the RH. Warming moist air, as is being down here, lowers the RH. Once back at the starting temperature, Point 5, the air now has a RH of only 10%.
Drying moist air is basically wringing moisture from a wet sponge.
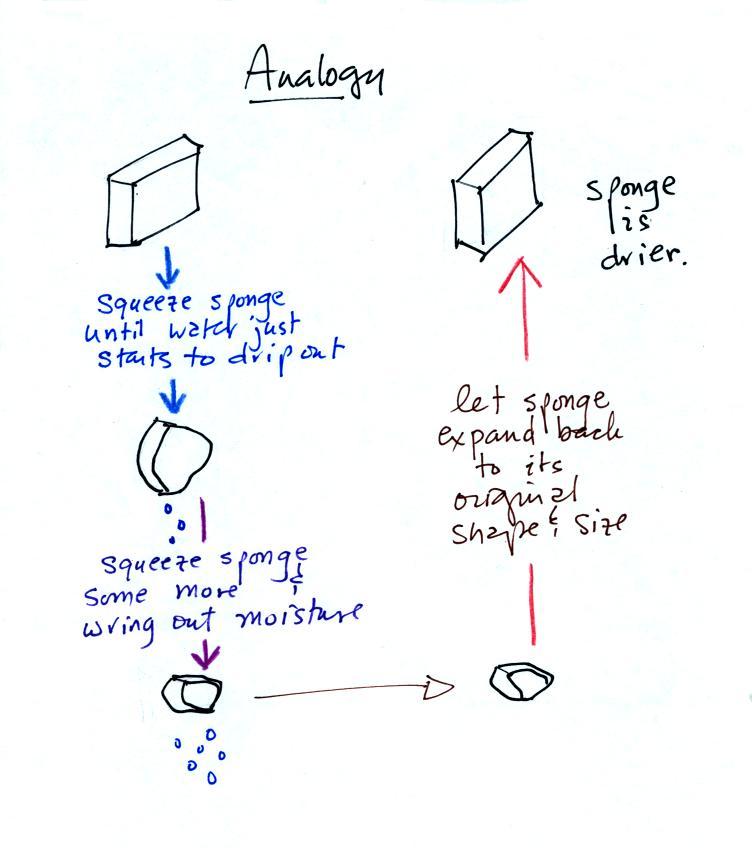
You start to squeeze the sponge and it gets smaller. That's like cooling the air and reducing the saturation mixing ratio, the air's capacity for water vapor. At first squeezing the sponge doesn't cause anything to happen (that's like cooling the air, the mixing ratio stays constant as long as the air doesn't lose any water vapor). Eventually water will start to drop from the sponge (with air this is what happens when you reach the dew point and continue to cool the air below the dew point). Then you let go of the sponge and let it expand back to its original shape and size (the air warms back to its original temperature). The sponge (and the air) will be drier than when you started.
Dry air indoors in the winter
The air indoors in the winter is often quite dry.
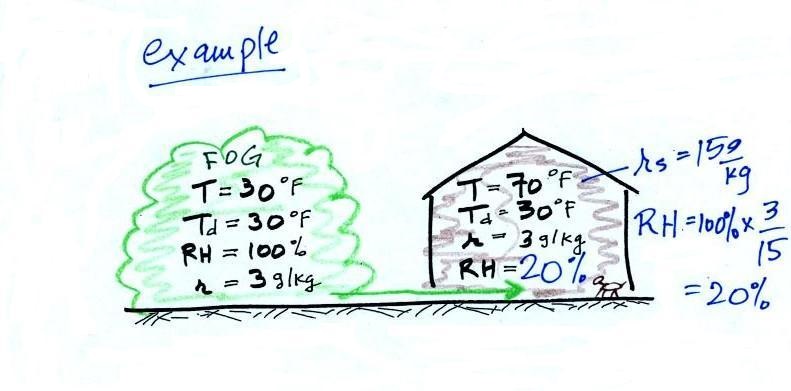
In the winter, cold air is brought inside your house or apartment and warmed. Imagine foggy 30 F air (with a RH of 100% this is a best case scenario, the cold air outdoors usually has a relative humidity less than 100% and is drier). Bringing the air inside and warming it will cause the RH to drop from 100% to 20%.. This can cause chapped skin, can irritate nasal passages, and causes cat's fur to become charged with static electricity.
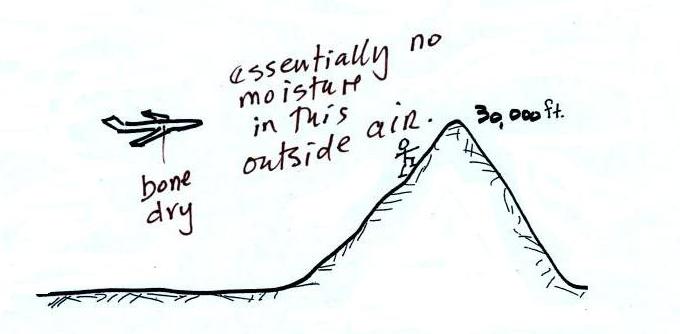
The air in an airplane comes from
outside the plane. The air outside the plane can
be very cold (-60 F perhaps) and contains very little
water vapor (even if the -60 F air is saturated it would
contain essentially no water vapor). When brought
inside and warmed to a comfortable temperature, the RH
of the air in the plane would be essentially 0%.
The RH doesn't get this low because the airplane adds
moisture to the air to make to make the cabin
environment tolerable. Still the RH of the air
inside the plane is pretty low and passengers often
complain of dehydration
on long airplane flights. This
may increase the risk of catching a cold (ref)
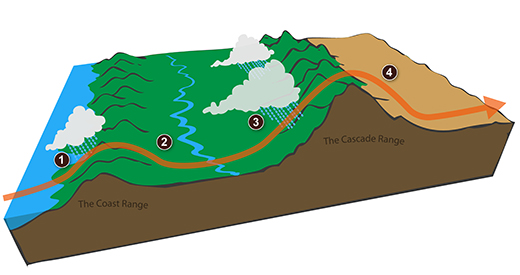
The rain-shadow effect
Next a much more important example of drying moist air (see p. 88 in the photocopied ClassNotes).
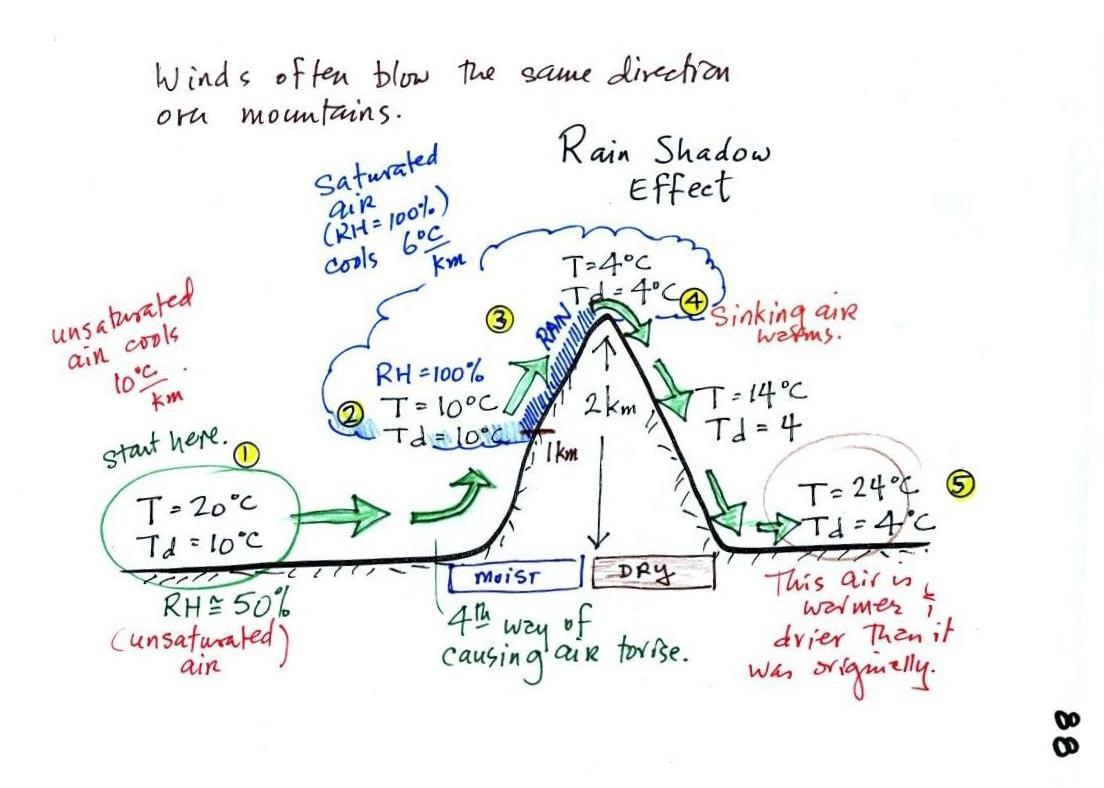
We start with some moist but unsaturated air (the RH is about 50%) at Point 1 (the air and dew point temperatures would need to be equal in order for the air to be saturated). As it is moving toward the right the air runs into a mountain and starts to rise (this is the 4th way of causing rising air motions). Rising air expands and cools. Unsaturated air cools 10 C for every kilometer of altitude gain (this is known as the dry adiabatic lapse rate but isn't something you need to remember). So after rising 1 km the air will cool to 10 C which is the dew point.
The air becomes saturated at Point 2 (the air temperature and the dew point are both 10 C). Would you be able to tell if you were outdoors looking at the mountain? Yes, you would see a cloud appear.
Now that the RH = 100%, the saturated air cools at a slower rate than unsaturated air (condensation of water vapor releases latent heat energy inside the rising volume of air, this warming partly offsets the cooling caused by expansion). We'll use a value of 6 C/km (an average value). The air cools from 10 C to 4 C in next kilometer up to the top of the mountain. Because the air is being cooled below its dew point at Point 3, some of the water vapor will condense and fall to the ground as rain. Moisture is being removed from the air and the value of the mixing ratio (and the dew point temperature) decreases.
At Point 4 the air starts back down the right side of the mountain. Sinking air is compressed and warms. As soon as the air starts to sink and warm, the relative humidity drops below 100% and the cloud disappears. The sinking unsaturated air will warm at the 10 C/km rate.
At Point 5 the air ends up warmer (24 C vs 20 C) and drier (Td = 4 C vs Td = 10 C) than when it started out. The downwind side of the mountain is referred to as a "rain shadow" because rain is less likely there than on the upwind side of the mountain. Rain is less likely because the air is sinking and because the air on the downwind side is drier than it was on the upslope side.
This is topographic lifting, the 4th of 4 processes that can cause air to rise. The other three were: convergence (surface winds spiraling inward toward a low pressure center will rise), fronts (both warm and cold fronts cause air to rise), and convection (warm air rises).
 |
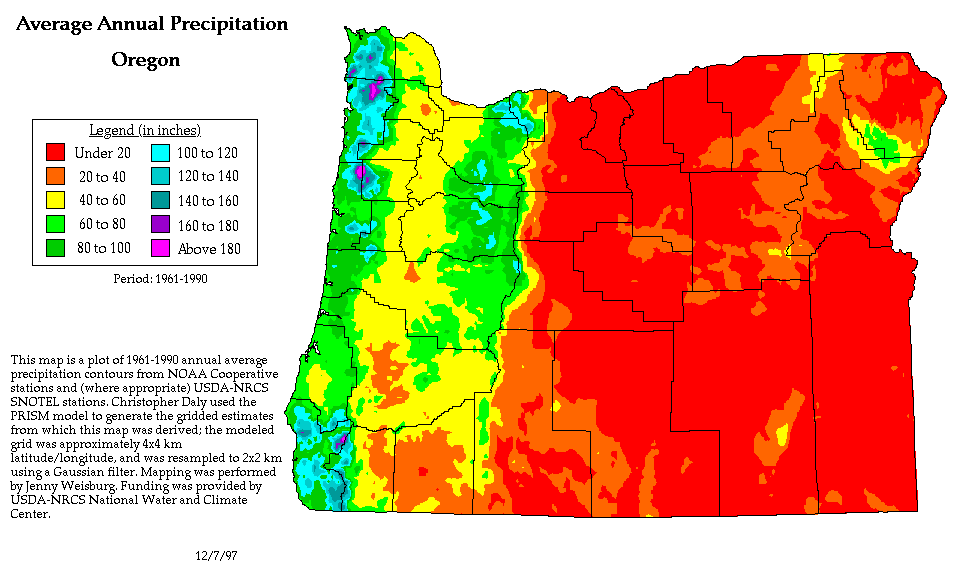 |
We can see the effects of a rain shadow illustrated well in the state of Oregon. The figure above at left shows the topography (here's the source of that map). Winds generally blow from west to east across the state.
Coming off the Pacific Ocean the winds first encounter a coastal range of mountains. On the precipitation map above at right (source) you see a lot of greens and blue on the western sides of the coastal range. These colors indicate yearly rainfall totals that range from about 50 to more than 180 inches of rain per year. Temperate rainforests are found in some of these coastal locations. The line separating the green and yellow on the left side of the precipitation map is the summit, the ridgeline, of the coastal mountain range.
That's the Willamette River valley, I think, in between the coastal range and the Cascades. This valley is somewhat drier than the coast because air moving off the Pacific has lost some of its moisture moving over the coastal range.
What moisture does remain in the air is removed as the winds move up and over the taller Cascades. The boundary between yellow/green and the red is the ridgeline of the Cascade Mountains. Yearly rainfall is generally less than 20 inches per year on the eastern side, the rain shadow side, of the Cascades. That's not too much more than Tucson which averages about 12 inches of rain a year.
 |
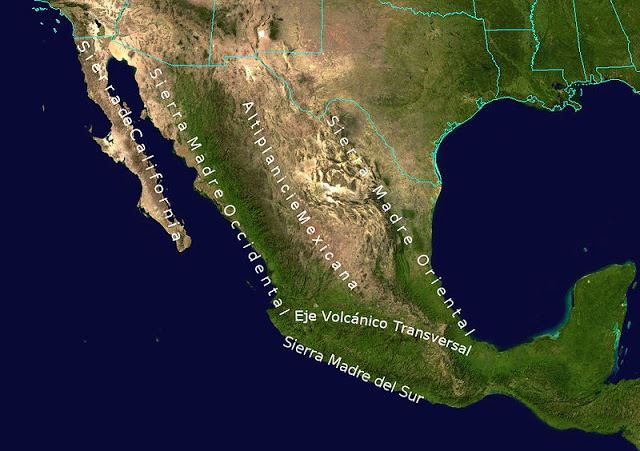 |
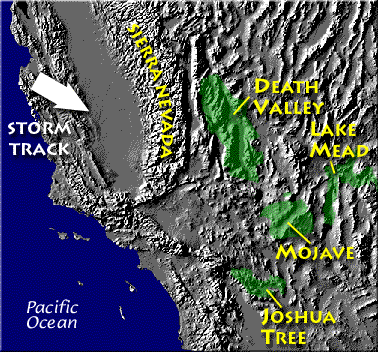
Death valley is found on the downwind side of the Sierra Nevada mountains (source of left image). The Chihuahuan desert and the Sonoran desert are found downwind of the Sierra Madre mountains in Mexico (source of the right image). Mexico might be a little harder to figure out because moist air can move into the interior of the country from the east and west at different times of the year. But there are mountains along both coasts, so some of that moisture will be removed before arriving in the center of the county.
Most of the year, the air that arrives in Arizona comes from the west, from the Pacific Ocean (this changes in the summer). It usually isn't very moist by the time it reaches Arizona because it has traveled up and over the Sierra Nevada mountains in California and the Sierra Madre mountains further south in Mexico. The air loses much of its moisture on the western slopes of those mountains. Beginning in early July in southern Arizona we start to get air coming from the south or southeast. This air can be much moister and leads to development of our summer thunderstorms.
Just as some of the world's driest regions are found on the downwind side (the rain shadow side) of mountain ranges, some of the wettest locations on earth are on the upwind sides of mountains. There seems to be some debate whether Mt. Wai'ale'ale in Hawaii or Cherrapunji India gets the most rain per year. Both get between 450 and 500 inches of rain per year.
Measuring humidity with a sling psychrometer
A short discussion of how you might try to measure humidity. One of the ways is to use a sling (swing might be more descriptive) psychrometer.
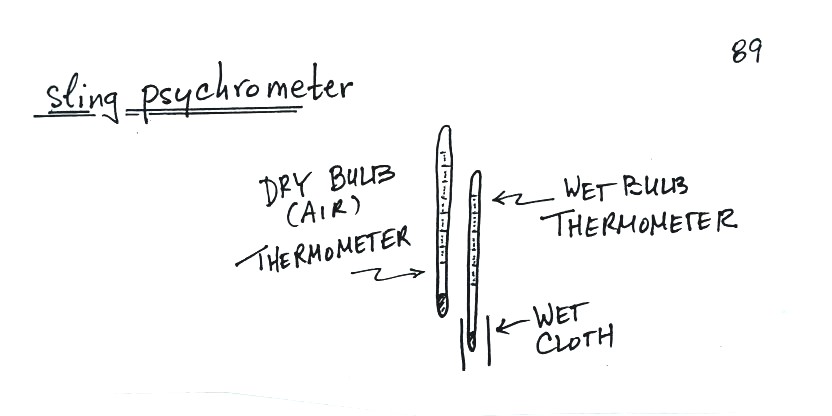
A sling psychrometer consists of two thermometers mounted side by side. One is an ordinary thermometer, the other is covered with a wet piece of cloth. To make a humidity measurement you swing the psychrometer around for a minute or two and then read the temperatures from the two thermometers. The dry thermometer measures the air temperature.
Would the wet thermometer be warmer or colder or the same as the dry thermometer? You can check it out for yourself - go get one of your hands wet. Does it feel the same as the dry hand? You might blow on both hands to increase the evaporation from the wet hand. I think you'll find the wet hand feels colder. That's what happens with the wet bulb thermometer.
 |
 |
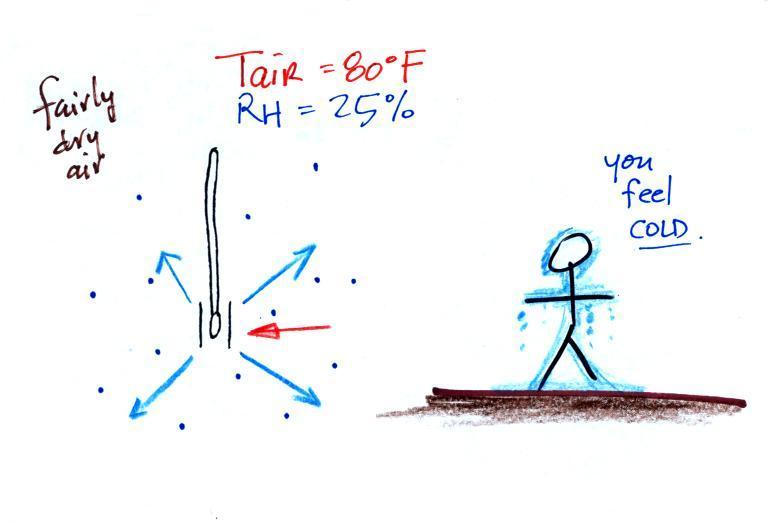
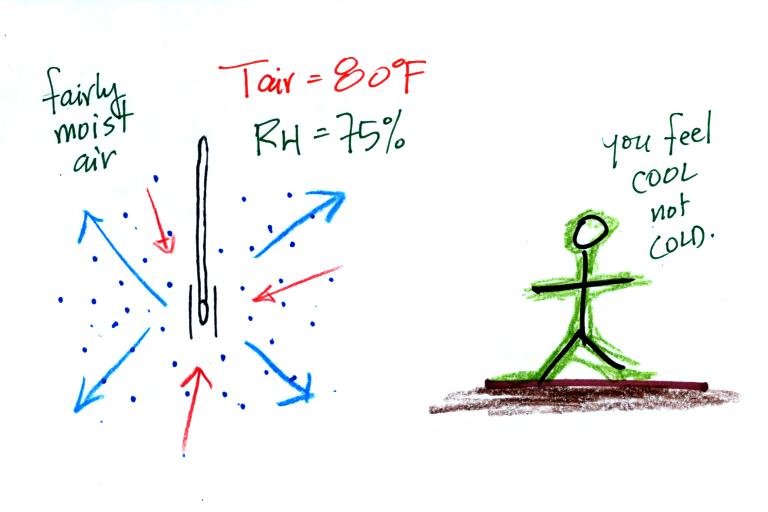
What could you say about the relative humidity in these two situations (you can assume the air temperature is the same in both pictures). You would feel coldest on a dry day (the left picture indicates dry air). The evaporative coolers that many people use in Tucson in the summer work much better (more cooling) early in the summer when the air is dry. Once the thunderstorm season begins in July and the air is more humid it is hard to cool your house below 80 F.
Here are a bunch of details that you can read through if you're so inclined. My goal is that you understand the basic principle behind a sling psychrometer. For that I think you can just skip to the summary a few pictures further on.
You need to be aware of a few things to understand the pictures that follow:
(1) warm water evaporates more rapidly than cold water
(2) whenever there is any moisture in the air, there will be some condensation. The rate of condensation will depend on how much moisture is in the air
(3) these two phenomena, evaporation and condensation, operate independently of each other
Here's the situation on a day with low relative humidity.
The evaporation is shown as blue arrows because this will cool the thermometer. The water on the wet thermometer starts out at 80 F and evaporates fairly rapidly.
The figure at upper left also shows one arrow of condensation. The amount or rate of condensation depends on how much water vapor is in the air surrounding the thermometer. In this case (low relative humidity) there isn't much water vapor. The condensation arrow is orange because the condensation will release latent heat and warm the thermometer.
Because there is more evaporation (4 arrows) than condensation (1 arrow) the wet bulb thermometer will drop. As the thermometer cools the rate of evaporation will decrease. The thermometer will continue to cool until the evaporation has decreased enough that it balances the condensation.
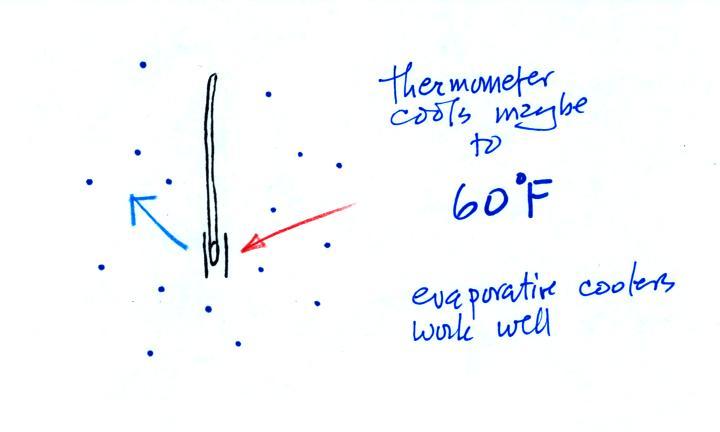
The rates of evaporation and condensation are equal. The temperature will now remain constant.
The figure below shows the situation on a day with higher relative humidity. There's enough moisture in the air to provide 3 arrows of condensation.
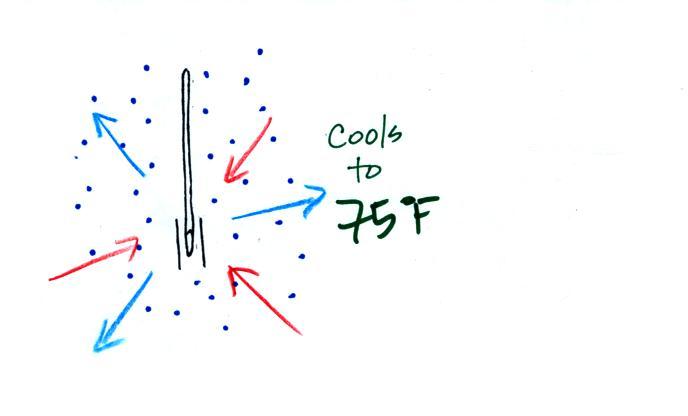
There'll only be a little cooling before the evaporation is reduced enough to be in balance with condensation.
Here's a visual summary
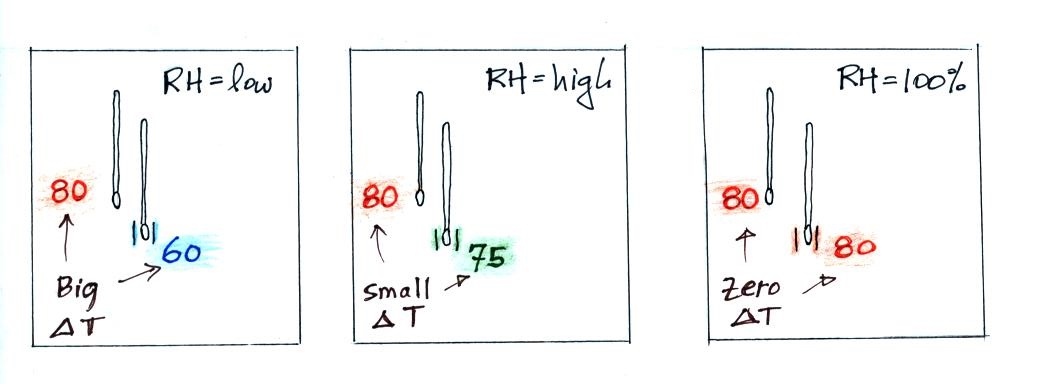
A large difference between the dry and wet temperatures means the relative humidity is low. A small difference means the RH is higher. No difference means the relative humidity is 100%.
We saw the same kind of relationship between RH and the difference between air and dew point temperature.
Wind chill and heat index
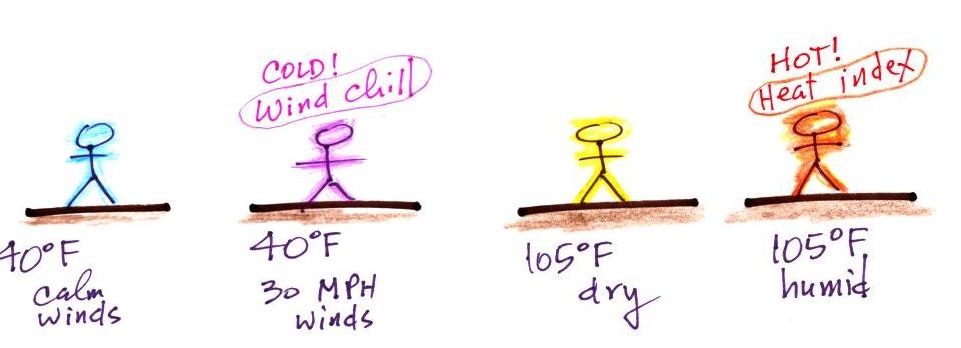
Cold temperatures and wind make it feel
colder than it really is. The wind
chill temperature tells you how much colder it will feel
( a thermometer would measure the same temperature on both the
calm and the windy day). If your body isn't able to keep
up with the heat loss, you can get hypothermia
and die.
There's something like that involving heat and humidity. High temperature and high humidity makes it feel hotter than it really is. Your body tries to stay cool by perspiring. You would feel hot on a dry 105 F day. You'll feel even hotter on a 105 F day with high relative humidity because your sweat won't evaporate as quickly. The heat index measures how much hotter you'd feel. The combination of heat and high humidity is a serious, potentially deadly, weather hazard because it can cause heatstroke (hyperthermia).
The drinking bird
Evaporative cooling and saturation are involved in the "drinking bird".
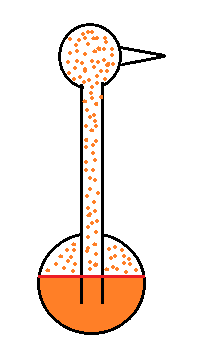
I'm very proud of the bird I found online. It is about twice as big as what you normally find. The bird is filled with a volatile liquid of some kind (ether?). Initially the bird's head and butt are the same temperature. The liquid inside the bird evaporates and saturates the air inside with vapor.
Next you get the bird's head wet. Instead of water I cheat a little bit and use isopropyl alcohol (rubbing alcohol) because it evaporates more rapidly than water. The evaporation of alcohol, just as with water, cools the bird's head.
As we saw last week, the saturation mixing ratio (saturation vapor concentration) of water depends on temperature. Warm air can contain more water vapor than colder air. The same applies to the ether vapor in this case. The head is still saturated with vapor but there is less vapor in the cool head than there is in warm saturated air in the bird's butt.
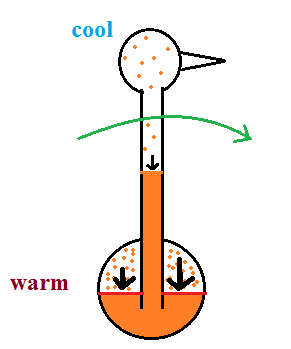
The differences in amounts of vapor produce pressure differences. The higher pressure at the bottom pushes liquid up the stem of the bird. The bird becomes top heavy and starts to tip.
At some point the bottom end of the stem comes out of the pool of liquid at the base. Liquid drains from the neck and the bird straightens up.
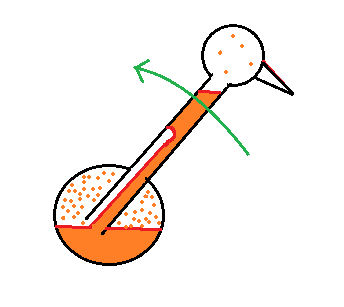
You can arrange the bird so that when it tips its beak dips into a small cup of water (or alcohol). This keeps the head moist and cool and the dipping motion could go on indefinitely. Here's a video.
We took away the bird's supply of alcohol, the bird warmed up and stopped tipping.
There's something like that involving heat and humidity. High temperature and high humidity makes it feel hotter than it really is. Your body tries to stay cool by perspiring. You would feel hot on a dry 105 F day. You'll feel even hotter on a 105 F day with high relative humidity because your sweat won't evaporate as quickly. The heat index measures how much hotter you'd feel. The combination of heat and high humidity is a serious, potentially deadly, weather hazard because it can cause heatstroke (hyperthermia).
The drinking bird
Evaporative cooling and saturation are involved in the "drinking bird".

I'm very proud of the bird I found online. It is about twice as big as what you normally find. The bird is filled with a volatile liquid of some kind (ether?). Initially the bird's head and butt are the same temperature. The liquid inside the bird evaporates and saturates the air inside with vapor.
Next you get the bird's head wet. Instead of water I cheat a little bit and use isopropyl alcohol (rubbing alcohol) because it evaporates more rapidly than water. The evaporation of alcohol, just as with water, cools the bird's head.
As we saw last week, the saturation mixing ratio (saturation vapor concentration) of water depends on temperature. Warm air can contain more water vapor than colder air. The same applies to the ether vapor in this case. The head is still saturated with vapor but there is less vapor in the cool head than there is in warm saturated air in the bird's butt.

The differences in amounts of vapor produce pressure differences. The higher pressure at the bottom pushes liquid up the stem of the bird. The bird becomes top heavy and starts to tip.
At some point the bottom end of the stem comes out of the pool of liquid at the base. Liquid drains from the neck and the bird straightens up.
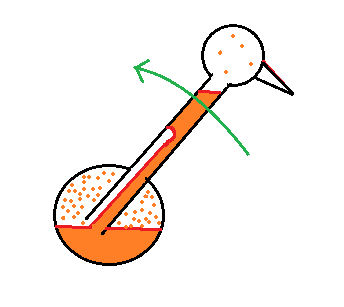
You can arrange the bird so that when it tips its beak dips into a small cup of water (or alcohol). This keeps the head moist and cool and the dipping motion could go on indefinitely. Here's a video.
We took away the bird's supply of alcohol, the bird warmed up and stopped tipping.
Condensation nuclei and the formation of dew, frost, haze, fog, and clouds
Here's a visual summary of a part of what we'll be covering next.
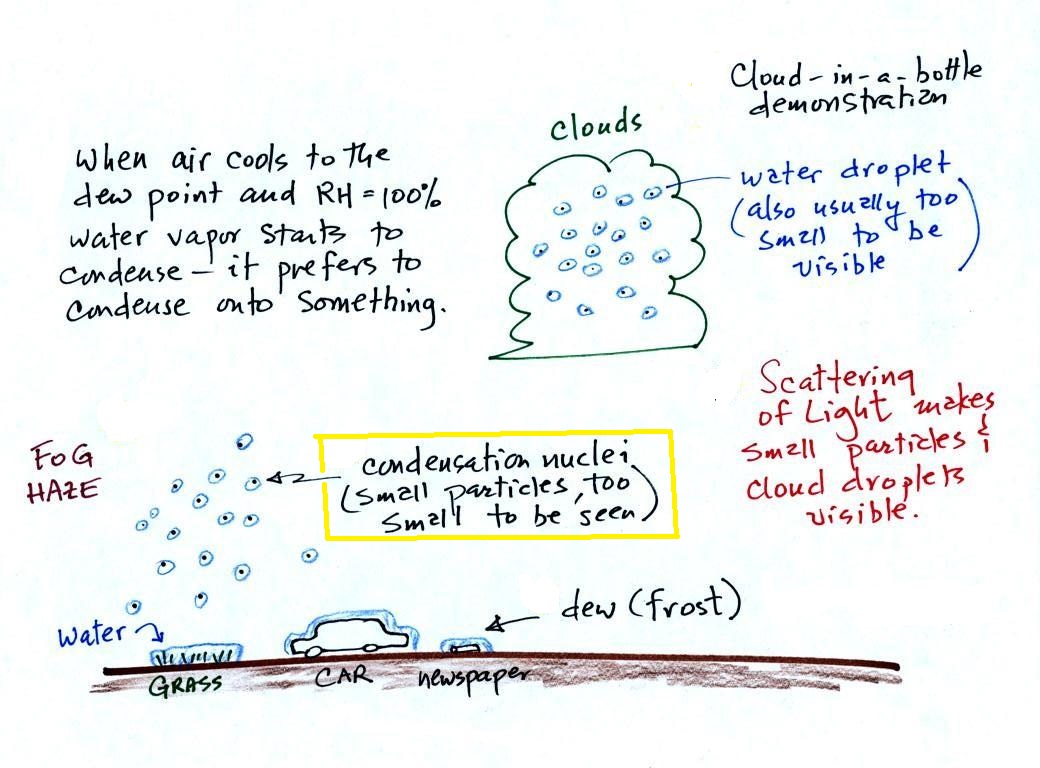
When air above the ground cools to the dew point, it is much easier for water vapor to condense onto small particles in the air called condensation nuclei. It would be much more difficult for the water vapor to condense and form small drops of pure water. Both the condensation nuclei and the small water droplets that form on them are usually too small to be seen with the naked eye. We can tell they are present because they scatter sunlight and make the sky hazy. As humidity increases dry haze turns to wet haze and eventually to fog. We'll try to make a cloud in a bottle and you'll be able to better appreciate the role that condensation nuclei play.
In the second half of the class we will begin to learn how to identify and name clouds.
Condensation nuclei and the role they play in cloud droplet formation
The air next to the ground cools during the night. Sometimes it cools enough to reach the dew point. Water vapor condenses onto objects on the ground and you find everything covered with dew (or frost) the next morning. When this happens in the air up above the ground you might think that water vapor would simply condense and form little droplets. This is not the case; we will find that small particles in the air called condensation play an essential role in cloud (and fog) formation.
| it is much
easier for water vapor to condense onto small particles called condensation nuclei |
it would
be much harder
for water vapor to just condense and form small droplets of pure water |
We didn't go into all of the details that follow in class, though they aren't hard to figure out and understand. If you'd prefer to just skip the details, just remember that particles make it easier for cloud droplets and clouds to form.
When the air is saturated with water vapor (the relative humidity is 100%) the rates of evaporation and condensation above a flat surface of water will be equal.

It's hard for water vapor to condense and form a small droplet of water because small droplets evaporate at a very high rate. This is known as the curvature effect and is illustrated below.

The surface of the smallest droplet above at left has the most curvature and the highest rate of evaporation (6 arrows). If a small droplet like this were to form, it wouldn't stay around very long. With it's high rate of evaporation it would quickly evaporate away and disappear.
The middle droplet is larger and would stick around a little longer because it does not evaporate as quickly. But it too would eventually disappear.
The drop on the right is large enough that curvature no longer has an effect. This drop has an evaporation rate (3 arrows) that is the same as would be found over a flat surface of water. A droplet like this could survive, but the question is how could it get this big without going through the smaller sizes with their high rates of evaporation. A droplet must somehow reach a critical size before it will be in equilibrium with its surroundings.
Particles in the air, cloud condensation nuclei (CCN), make it much easier for cloud droplets to form. The figure below explains why.

There are always lots of CCN (cloud condensation nuclei in the air) so this isn't an impediment to cloud formation. The following information is from p. 91 in the ClassNotes.
Now back to material that we did cover in class.

Note that condensation onto
certain kinds of condensation nuclei and growth of cloud
droplets can begin even when the relative humidity is below
100%. These are called hygroscopic nuclei.
Salt is an example; small particles of salt mostly come
from evaporating drops of ocean water.
Here are some more of the details that we didn't cover in class. To understand how this can occur we first need to learn about the solute effect
Here are some more of the details that we didn't cover in class. To understand how this can occur we first need to learn about the solute effect
 |
 |
| solution droplet |
pure water droplet |
The next figure compares solution droplets that form when the RH is 100% (left figure) and when the RH is less than 100%.
 |
 |
| the droplet is able to
grow |
the droplet is in
equilibrium with its surroundings even when the RH is less than 100% |
The solution droplet will grow in the RH=100% environment at left. You can tell the RH is less than 100% in the figure at right because there are now only 2 arrows of evaporation. But because the solution droplet only has 2 arrows of evaporation it can form and be in equilibrium in this environment.
Back again to material covered in class
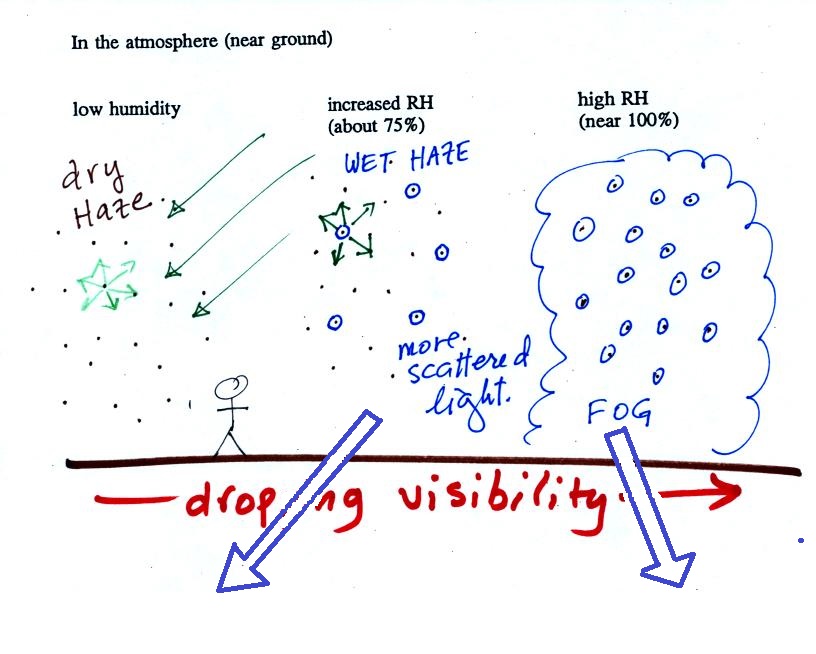
The following figure is at the bottom of p. 91 in the ClassNotes and illustrates how cloud condensation nuclei and increasing relative humidity can affect the appearance of the sky and the visibility.
The air in the left most figure is relatively dry. Even though the condensation nuclei particles are too small to be seen with the human eye you can tell they are there because they scatter sunlight. When you look at the sky you see the deep blue color caused by scattering of sunlight by air molecules mixed together with some white sunlight scattered by the condensation nuclei. This changes the color of the sky from a deep blue to a bluish white color. The more particles there are the whiter the sky becomes. This is called "dry haze." Visibility under these conditions might be anywhere from a few miles up to a few tens of miles.
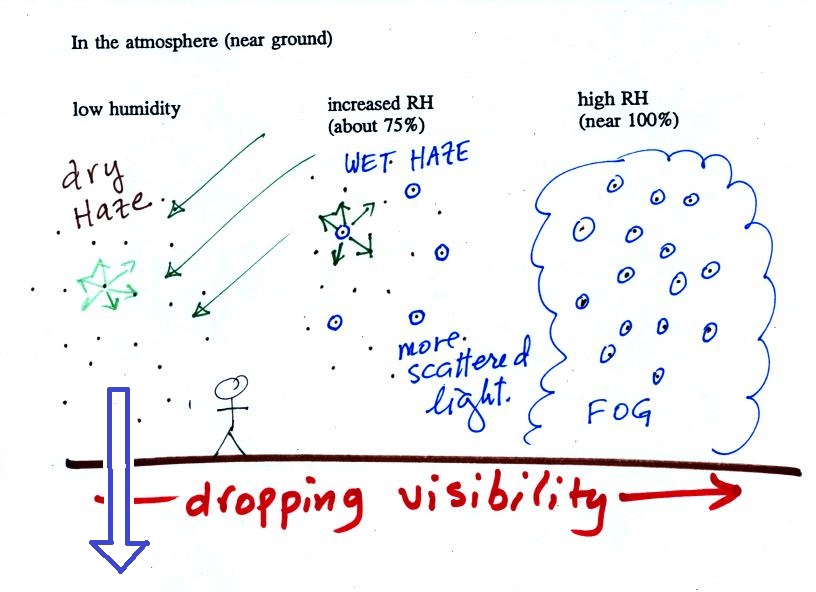

The middle picture below shows what happens when you drive from the dry southwestern part of the US into the humid southeastern US or the Gulf Coast. One of the first things you would notice is the hazier appearance of the air and a decrease in visibility. It isn't that there are more particles. The relative humidity is higher, water vapor begins to condense onto some of the condensation nuclei particles (the hygroscopic nuclei) in the air and forms small water droplets. The water droplets scatter more sunlight than just small particles alone. The increase in the amount of scattered light is what gives the air its hazier appearance. This is called "wet haze." Visibility now might now only be a few miles.
 |
 |
| Thin
fog (perhaps even wet haze) with pretty good visibility (source of the image) |
Thick
fog (visibility was less than 500 feet) (source of the image) |
Finally when the relative humidity increases to 100% fog forms and water vapor condenses onto all the condensation nuclei. Fog can cause a severe drop in the visibility. The thickest fog forms in dirty air that contains lots of condensation nuclei. That is part of the reason the Great London Smog of 1952 was so impressive. Visibility was at times just a few feet!
This is as far as we were able to get in class today. I'll postpone the Cloud-in-a-bottle demonstration until next Tuesday.
Making a cloud in a bottle
Cooling air & increasing relative humidity, condensation nuclei, and scattering of light
are all involved in this demonstration.
Cooling air & increasing relative humidity, condensation nuclei, and scattering of light
are all involved in this demonstration.
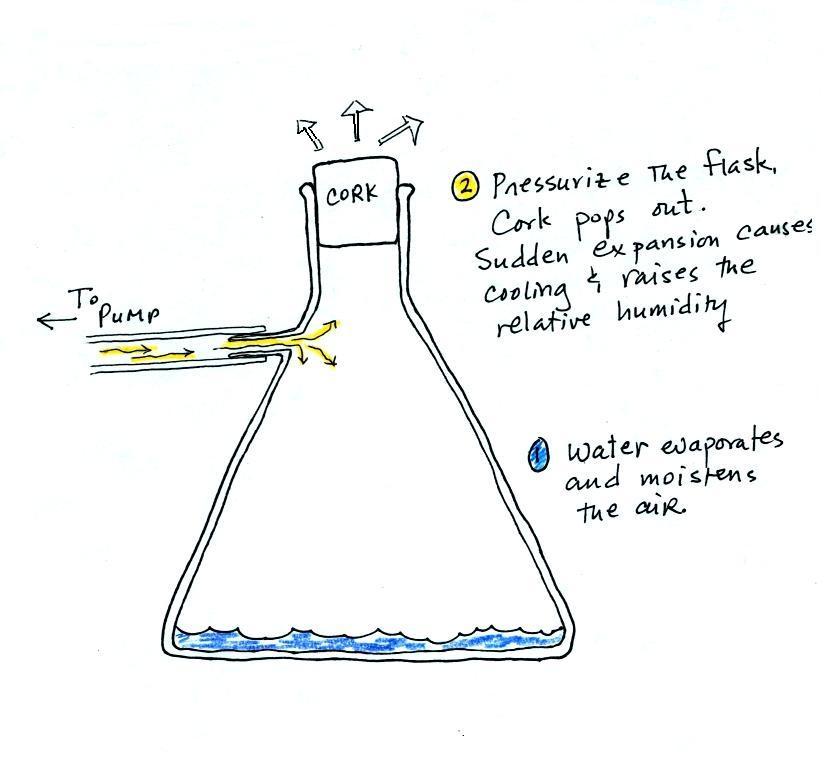
We used
a strong, thick-walled, 4 liter vacuum flask
(designed to not implode when all of the air is
pumped out of them, they really aren't designed to
be pressurized). There was a little water in
the bottom of the flask to moisten the air in the
flask. Next we pressurized the air in the
flask with a bicycle pump. At some point the
pressure blows the cork out of the top of the
flask. The air in the flask expands outward
and cools. This sudden cooling increases the
relative humidity of the moist air in the flask to
more than 100% momentarily and water vapor
condenses onto cloud condensation nuclei in the
air.
I like it best when a faint, hard to see, cloud becomes visible. That's because there is something we can add to the demonstration that will make the cloud much "thicker" and easier to see.
I like it best when a faint, hard to see, cloud becomes visible. That's because there is something we can add to the demonstration that will make the cloud much "thicker" and easier to see.
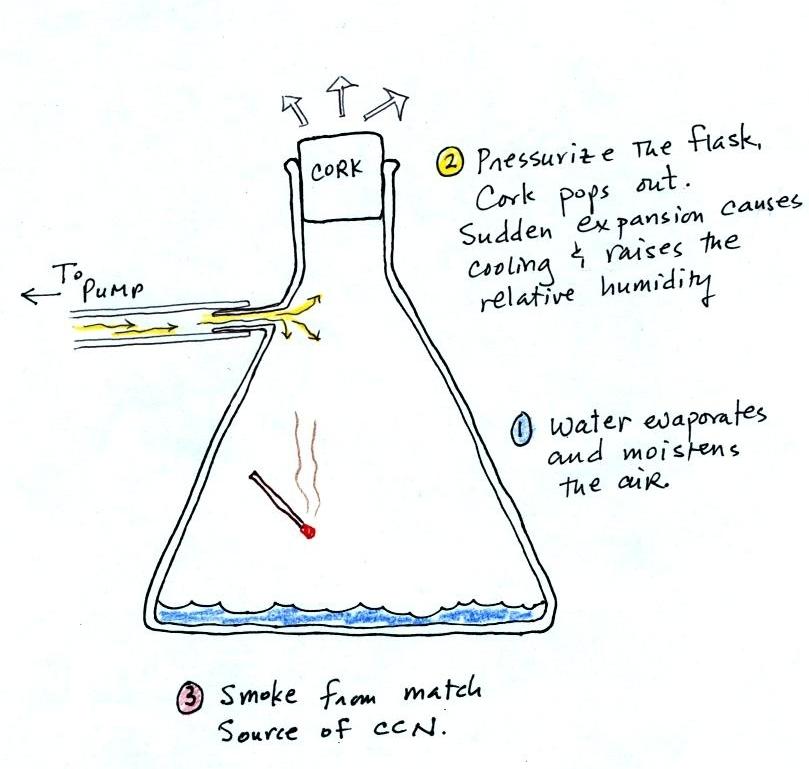
The demonstration was repeated an
additional time with one small change. A burning match
was dropped into the bottle. The smoke from the matches
added lots of very small particles, condensation nuclei, to
the air in the flask (you could see the swirls of smoke, the
small particles scattered light). The same amount of
water vapor was available for cloud formation but the cloud
that formed this time was quite a bit "thicker" and much
easier to see. To be honest the burning match probably
also added a little water vapor (water vapor together with
carbon dioxide is one of the by products of combustion).
I have found a couple of online versions of the demonstration. The first is performed by Bill Nye "The Science Guy" and is pretty similar to the one done in class. The second differs only in the way that is used to caused the sudden expansion and cooling of the air (I didn't care much for the music (probably your opinion of the music I play before class) and would recommend turning down the sound while watching the video).
Clouds and climate change
This effect has some implications for climate change.
I have found a couple of online versions of the demonstration. The first is performed by Bill Nye "The Science Guy" and is pretty similar to the one done in class. The second differs only in the way that is used to caused the sudden expansion and cooling of the air (I didn't care much for the music (probably your opinion of the music I play before class) and would recommend turning down the sound while watching the video).
Clouds and climate change
This effect has some implications for climate change.
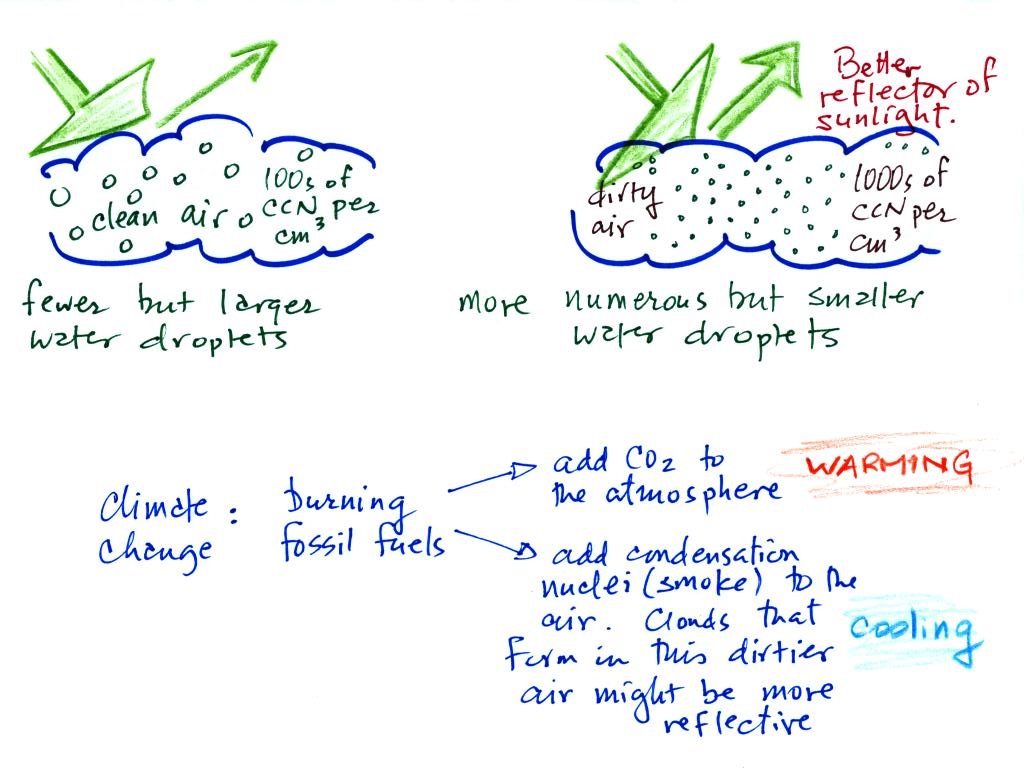
A cloud that forms in
dirty air is composed of a large number of small droplets
(right figure above). This cloud is more reflective than
a cloud that forms in clean air, that is composed of a smaller
number of larger droplets (left figure).
Combustion of fossil fuels adds carbon dioxide to the atmosphere. There is concern that increasing carbon dioxide concentrations (and other greenhouse gases) will enhance the greenhouse effect and cause global warming. Combustion also adds condensation nuclei to the atmosphere (just like the burning match added smoke to the air in the flask). More condensation nuclei might make it easier for clouds to form, might make the clouds more reflective, and might cause cooling. There is still quite a bit of uncertainty about how clouds might change and how this might affect climate. Remember that clouds are good absorbers of IR radiation and also emit IR radiation.
Clouds are one of the best ways of cleaning the atmosphere. This is something we mentioned earlier in the semester and you're now in a position to understand it better.
Combustion of fossil fuels adds carbon dioxide to the atmosphere. There is concern that increasing carbon dioxide concentrations (and other greenhouse gases) will enhance the greenhouse effect and cause global warming. Combustion also adds condensation nuclei to the atmosphere (just like the burning match added smoke to the air in the flask). More condensation nuclei might make it easier for clouds to form, might make the clouds more reflective, and might cause cooling. There is still quite a bit of uncertainty about how clouds might change and how this might affect climate. Remember that clouds are good absorbers of IR radiation and also emit IR radiation.
Clouds are one of the best ways of cleaning the atmosphere. This is something we mentioned earlier in the semester and you're now in a position to understand it better.
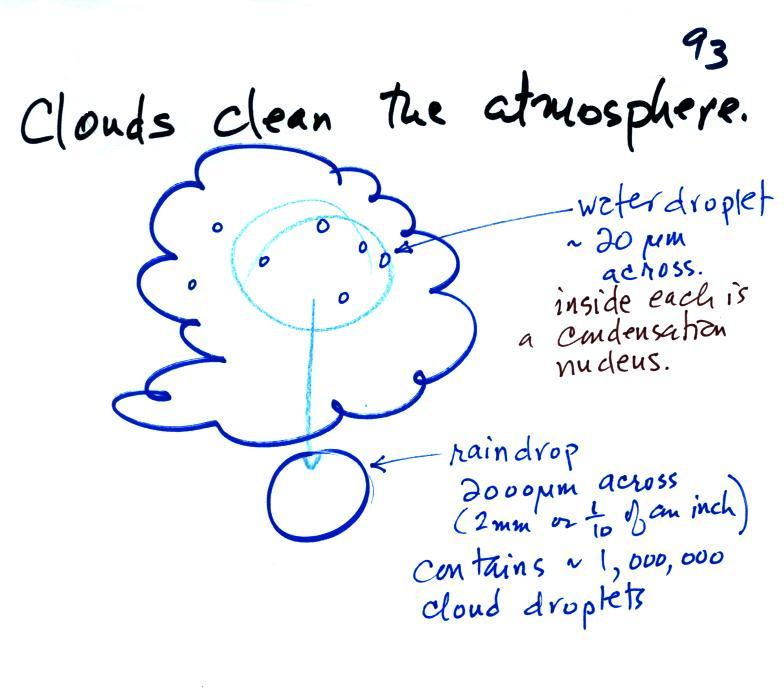
A cloud is composed of small water droplets (diameters of 10 or 20 micrometers) that form on particles ( diameters of perhaps 0.1 or 0.2 micrometers). The droplets "clump" together to form a raindrop (diameters of 1000 or 2000 micrometers which is 1 or 2 millimeters), and the raindrop carries the particles to the ground. A typical raindrop can contain 1 million cloud droplets so a single raindrop can remove a lot of particles from the air. You may have noticed how clear the air seems the day after a rainstorm; distant mountains are crystal clear and the sky has a deep blue color. Gaseous pollutants can dissolve in the water droplets and be carried to the ground by rainfall also. We'll be looking at the formation of precipitation in more detail later this week.