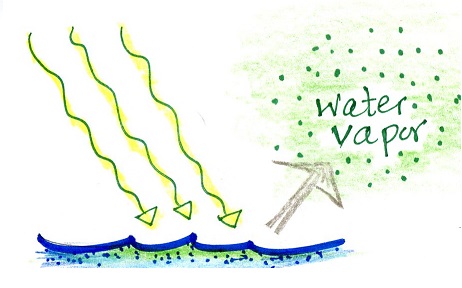
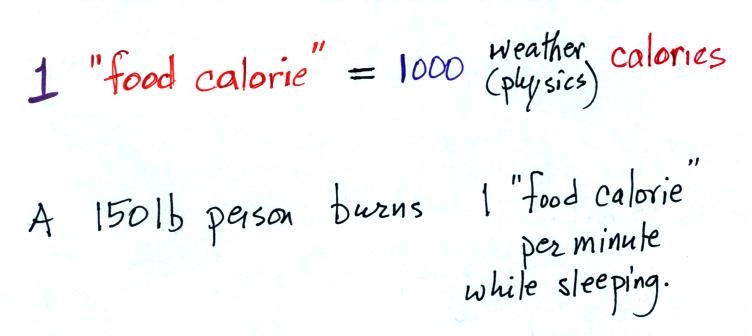Here are three examples showing
energy originally hidden in water vapor reemerging in a
tornado, water rushing down a mountain or wash, and a
hurricane. A big part of the energy in the tornado,
flash flood, and hurricane was initially hidden in the
water vapor.
2. Energy units
Next just brief mention of units of energy

Joules
are the units of energy that you would probably
encounter in a physics class. Your
electric bill shows the amount of energy that
you have used in a month's time, the units are
kilowatt-hours.
We'll usually be using calories as units of
energy. 1 calorie is the energy need to warm 1
gram of water 1 C (there are about 5 grams of water in
a teaspoon).
Here's a little miscellaneous information that you
don't need to worry about remembering. You've
probably seen the caloric content of food on food packages
or on menus in restaurants. 1 "food calorie" is
actually 1000 of the calories mentioned above (food is
probably a form of chemical energy, the energy is released
when the food is consumed).
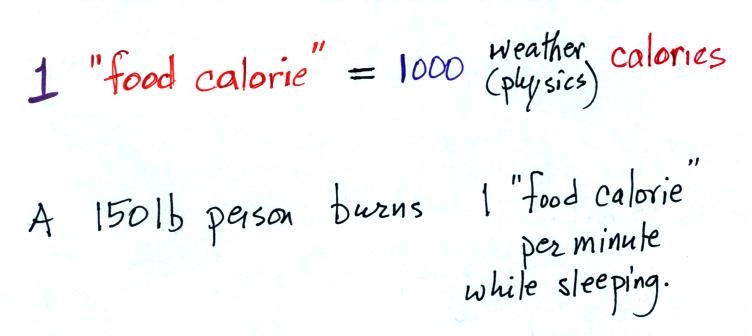
A 150 pound person would burn
almost 500 food calories while sleeping during the
night (8 hours x 60 minutes per hour x 1 food
calorie per minute). This is about the energy
contained in one donut.
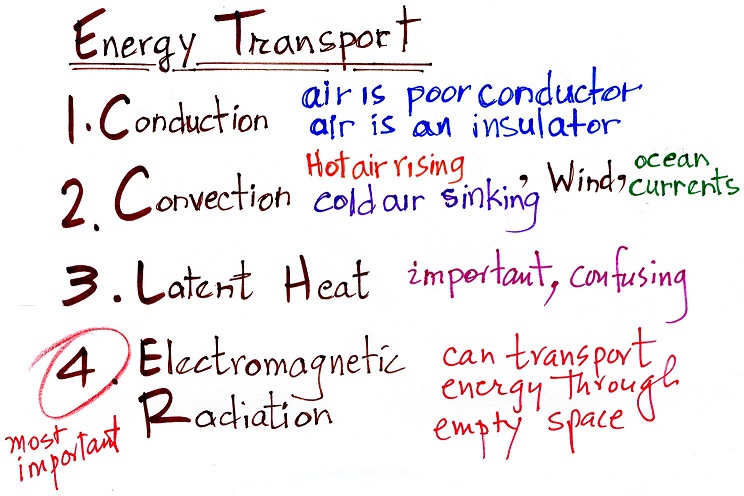
3. Energy transport processes
By far the most important process is at
the bottom of the list above. Energy transport in the
form of electromagnetic radiation (sunlight for example) is
the only process that can transport energy through empty
space. Electromagnetic radiation travels both to the
earth (from the sun) and away from the earth back into
space. Electromagnetic radiation is also responsible
for about 80% of the energy transported between
the ground and atmosphere.
You might be surprised to learn that latent heat is the
second most important transport process. This term
latent heat can refer to both a type of energy and an energy
transport process (the energy is hidden in the water vapor,
the water vapor can move around and carry that energy with
it).
Rising parcels of warm air and sinking parcels of cold
air are examples of free convection. Because of
convection you feel colder or a cold windy day than on a
cold calm day (the wind chill effect). Ocean
currents are also an example of convection.
Convection is also one of the ways of rising air motions
in the atmosphere (convergence into centers of low pressure
and fronts are two other ways we've encountered so
far)
Conduction is the least important energy transport at
least in the atmosphere. Air is such a poor conductor
of energy that it is generally considered to be an
insulator.