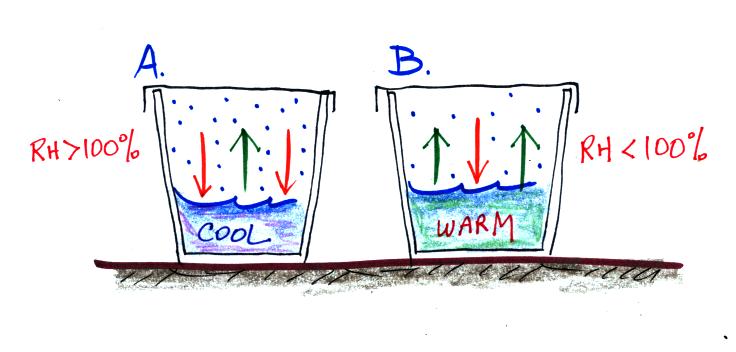
1. Is the water in Glass A WARMER or
COLDER than in Glass B?
The water in Glass A is COLDER
than in Glass B. How can you tell? The water in Glass A is
evaporating more slowly than in Glass B (1 arrow in A versus 2 arrows in B).
2. Is there MORE or LESS water vapor in the air in
Glass A than in Glass B?
There is MORE water vapor in
the air in Glass A than in Glass B. Water vapor is condensing more
rapidly in Glass A than in Glass B. The rate of condensation depends on
how much water vapor is in the air. There must be more water vapor in the
air in Glass A in order to provide the higher rate of condensation.
3. Is the relative humidity in each glass MORE than 100%, LESS than 100% or is
it EQUAL to 100%.
The relative humidity in Glass A is more than 100%
(the air is supersaturated). Because the rate of condensation in Glass A
is greater than the rate of evaporation, the rate of condensation in Glass A
will decrease. It will continue to decrease until it is equal to the rate
of evaporation.
The relative humidity in Glass B is less than 100%
(the air is subsaturated). The higher rate of
evaporation will increase the amount of water vapor until condensation is able
to increase and balance the rate of evaporation.
4. The rates of evaporation and condensation aren't equal in either glass, so
the pictures will change with time. What will the glasses look once they
have reached equilibrium?
The relative humidity in Glass B is less than 100% (the air is subsaturated). There is more evaporation than
condensation. The amount of water vapor in Glass B will increase until
there is enough water vapor to provide 2 arrows of condensation which will
balance the rate of evaporation.

This is what the two glasses will look like once they've
achieved equilibrium. The air in both glasses is now saturated and the RH
= 100%. The air in Glass B, the warmer glass, contains more water vapor.