![[Home]](../../icons/home.jpg)
![[Lectures]](../../icons/lectures.jpg)
![[Previous]](../../icons/previous.jpg)
![[Next]](../../icons/next.jpg)
Composition of the atmosphere
The Earth's atmosphere is mostly composed of a mixture of gases with very tiny quantities
of aerosols, which are solid or liquid particles suspended in the air.
You should understand that most of the gases in the atmosphere are nitrogen
and oxygen. These will be referred to as the major components of the atmosphere. You should also
realize that more than half of Earth's atmosphere is composed of nitrogen (roughly 78%) with
comparatively less oxygen (about 21% of the atmosphere). The remaining gases in Earth's atmosphere are called trace
gases because these gases make up a very small percentage of the total.
By far the most abundant of these trace gases is argon (close to 1% of the total).
Even though a small percentage of the total atmosphere, there are hundreds of trace gases
in Earth's atmosphere, and some of them are absolutely essential for life as we know it.
Some of the trace gases are listed in the table below. Notice in the pie chart
that argon, carbon dioxide, and all the other trace gases, except water vapor, make up
a very small slice. In fact the concentrations on the right side of the table are given in units of parts per million (ppm),
where each ppm means one out of every 1 million molecules. For example, the current concentration
of carbon dioxide is about 400 ppm, which means that for every 1,000,000 gas molecules
only 400 of them are carbon dioxide.
The table below breaks up gases into permanent and variable categories as well. This is
to indicate that the concentrations variable gases change with time. The most variable of these is water vapor,
which is the gas form of water (literally molecules of H2O moving around with the rest of the gases
in the atmosphere). Water vapor only averages about 0.4% of the total gases, however, it varies quite a lot
depending on time and place, and can be over 2% of the total under warm, humid conditions. Obviously in
places where water vapor is high, the percentage of all other gases must go down some. This is indicated
in the pie chart below. You should be familar with some of the features of the important trace gases
described in the text below.
Composition of the Atmosphere near the Earth's
Surface
| Permanent Gases |
Variable Gases |
| Gas Name |
Chemical
Formula |
Percent
(by Volume)
Dry Air |
Gas
(and Particles) |
Symbol |
Percent
(by Volume) |
Parts per
Million (ppm)* |
| Nitrogen |
N2 |
78.08 |
Water Vapor |
H2O |
0 to 4 |
|
| Oxygen |
O2 |
20.95 |
Carbon Dioxide |
CO2 |
0.0400 |
400 |
| Argon |
Ar |
0.93 |
Methane |
CH4 |
0.00017 |
1.7 |
| Neon |
Ne |
0.0018 |
Nitrous Oxide |
N2O |
0.00003 |
0.3 |
| Helium |
He |
0.0005 |
Ozone |
O3 |
0.000004 |
0.04 |
| Hydrogen |
H2 |
0.00005 |
Particles (dust, soot, etc.) |
|
0.00001 |
0.01-0.15 |
| Xenon |
Xe |
0.000009 |
Chlorofluorocarbons (CFCs) |
|
0.00000002 |
0.0002 |
|
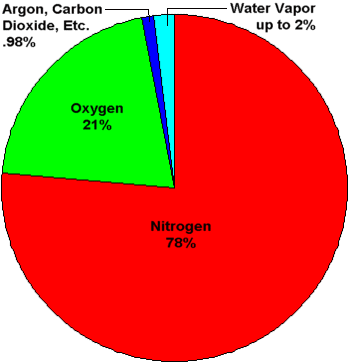
Pie chart showing percentage concentatrations of gases in Earth's atmosphere. Water vapor
is shown as a slice that can be up to 2% of the total. The concentration of water vapor
is highly variable and ranges from near 0% to over 2%. Averaged throughout the entire
atmosphere, water vapor makes up about 0.4% of the total.
|
Major Components of the Atmosphere
The Atmosphere has two main components: nitrogen(78%) and oxygen(21%).
These make up 99% of the volume of "dry
air". In this context "dry air" refers to all gases, except water vapor. Remember that even under the most
humid conditions on Earth, water vapor is at most 2% of the atmosphere. Thus, if you were an alien
studying the planet earth, you would report that Earth's atmosphere is mostly nitrogen and oxygen.
The text below mentions the main ways that nitrogen and oxygen gas are removed from the atmosphere
and enter the atmosphere as part of chemical cycles.
Nitrogen:
Removed from atmosphere by biological processes that
involve soil bacteria. Returned to the atmosphere through the
decaying of plant and animal matter.
Oxygen:
Removed from atmosphere by when organic matter decays,
combines with other substances, or is taken in during
breathing. Is added to the atmosphere through photosynthesis by
plants.
Some Important Trace Components of the Atmosphere
Trace gases by definition are scarce in Earth's atmosphere. Yet several of these trace
gases are essential for the life that has developed on Earth.
Water Vapor:
- The gas phase of water. Water vapor is literally individual molecules of H2O
that are part of the collection of gases in the atmosphere.
- Varies greatly from place to place, and from time to
time. It averages only about 0.4% of the atmosphere, but varies
from as much as 4% in the humid tropics to near 0% in cold polar regions.
- Enters the atmosphere through evaporation of liquid water.
- Water vapor condenses into liquid and solid cloud particles that grow in
size and fall to earth as precipitation
- Redistributes heat energy on earth and is important to
the formation of storms. This is because large quantities of
energy are involved in phase changes:
- Evaporation (liquid to gas) energy is absorbed from environment
- Condensation (gas to liquid) energy is released to the environment
- Is a strong
greenhouse
gas that warms the earth's surface and its atmosphere. In fact
water vapor is the most important greenhouse gas on Earth in that it contributes
most to the atmospheric
greenhouse effect.
Carbon Dioxide:
- Second most important greenhouse gas on Earth.
- Enters the atmosphere through the decay of vegetation,
volcanic eruptions,
respiration, burning of fossil fuels, and from
deforestation. It is removed from the atmosphere by
photosynthesis, and the oceans.
- Concentration has been increasing due to human activities, mainly buring fossil
fuels and deforestation. The amount of carbon dioxide has increased over 42%
since 1750, from 280 ppm to 400 ppm.
- There is concern that this will strengthen the natural greenhouse
effect leading to global warming, sea level rise, and other potentially harmful climate changes.
Methane:
- Another greenhouse gas that is increasing due
to human activity. There is concern that the increasing amount of methane will
also contribute to human caused global warming.
- Since 1750, methane concentrations have increased by more than
150% mainly due to human activity.
- The main sources are the breakdown of plant material in
rice paddies, domestic grazing animals (biological reactions
in their stomach), biological activities of termites.
Nitrous Oxide:
- Another important greenhouse gas. that is increasing due
to human activity. There is concern that the increasing amount of nitrous oxide will
also contribute to human caused global warming.
- Since 1750, nitrous oxide conntrations have increased by more than
20% mainly due to human activity.
- Forms in the soil by bacterial processes and is destroy by
ultraviolet light from the sun.
Ozone:
- Most ozone is found in the
stratosphere where it forms the ozone layer
(~20 - 30 km above the ground surface). The ozone layer
protects plants, animals, and humans from the sun's harmful
ultraviolet radiation by absorbing the radiation.
- Very little is found naturally near the ground where it
is a toxic pollutant. Sometimes dangerously high concentrations
develop near large cities in a process called
photochemical
smog
Aerosols:
Aerosols
are tiny solid or liquid particles that are suspended in the air. Most
aerosols are microscopic and too small to see individually without a microscope.
Aerosols include things like dust, pollen, smoke, and even cloud droplets. When there
are high concentrations of aerosols in the air, they do affect the propagation of
light, and thus affect visibility. Examples are the visible thick smoke that comes
off fires and normal clouds.
- Important for climate naturally and through human activities that
release aerosols into the atmosphere
- Affect passage of solar radiation through the atmosphere
- Influence cloud formation
- Natural and manmade aerosols can affect human health
- "Particulate" air pollution
![[Home]](../../icons/home.jpg)
![[Lectures]](../../icons/lectures.jpg)
![[Previous]](../../icons/previous.jpg)
![[Next]](../../icons/next.jpg)
