Tentative NATS 101 Quiz 1
Study Guide
You will be tested on material on the Practice
Quiz Study Guide
together with the following new material:
Ideal gas law
applications. If you heat or cool a parcel of air in the
atmosphere, the density
(volume)
will change in such a way that the air pressure inside the parcel
remains constant (remains the same as the pressure of the air
surrounding the parcel).
Upward and downward forces act on air parcels (the
strength of one of the forces depends on the air inside the parcel, the
other on the air outside the parcel). These two forces are usually in
balance. What happens to the balance when you warm or cool a parcel of
air? Basically you should be able to explain why a balloon of hot
low density air rises and a balloon of cold high density air sinks.
Sample question: Quiz 1: 16
***
Chap. 12 (pps 325-327, 330) ***
Stratospheric Ozone and the
Ozone
Hole. Natural production and
destruction of ozone. Importance of the ozone layer. Manmade
destruction of ozone. What part of a CFC molecule destroys ozone?
Ozone hole: what, where, when, and how does it form?
Sample questions: Final: 13, 15
***
Chap. 1 (pps 13-18), Chap. 6 (pps 141-149), Appendix C (pps 431-432)
***
Station model notation.
Cloud cover, temperature, dew point temperature (typical values for
Tucson), wind direction and speed, common weather symbols (rain, snow,
fog, rain shower, thunderstorm, tropical storm and hurricane),
pressure. Units. After pressure is measured, what important adjustment
is made before the pressure is plotted on the surface map? Why is that
necessary? Average value and typical range of sea-level pressures.
Surface weather maps.
Surface observations are made and a new map is prepared hourly. What
time zone or time reference is used? 24-hour clock (what time is it
when it is 17:30 in Tucson). Isobars and isotherms. Small horizontal
differences in pressure cause the wind to blow. Air motions around high
and low pressure centers (northern hemisphere). Strong and weak
pressure gradients. Convergence and divergence. Rising and sinking air
motions. How do wind motions around highs and low affect the
temperature pattern? Cold fronts and warm fronts.
Sample questions: Practice
Quiz: 3, 4, 7, 15, 16 Quiz 1:
1, 15, EC1. Final: 7, 49, 51
|
|
Upper level charts
(see pps
115-119 in the photocopied notes).
Ridges
(warm air below) and troughs (cold air below). Winds blow parallel to
contour lines and from west to east. Would upper-level convergence
cause surface pressure to increase or decrease?
Pressure decreases with increasing altitude in the
atmosphere. Does pressure decrease more quickly in warm or cold air,
in high or low density air? Why? Are upper level conditions normally
displayed on constant altitude or constant pressure charts?
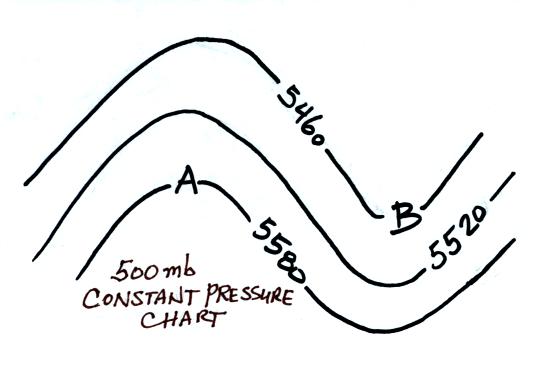
What do the numbers on the contour lines on the constant pressure
(isobaric) map above at left represent? Is the coldest air
found in the north or
south? Is the air below Point A warmer or colder than
the air below Point B. Is Point A in a ridge or a trough?
Is this a northern or a southern hemisphere
chart? Is the pressure at Point A on the map higher, lower,
or the same as the pressure at Point B? How do the altitudes at the
two points compare?
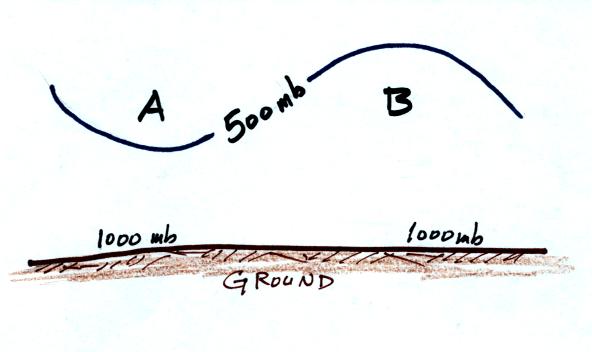
How does the pressure at point A in the crossectional figure above at
right compare with the pressure at Point B? Is the coldest air
found on the right or left hand side of the figure? Is pressure
decreasing most rapidly with increasing altitude on the right or left
side of the figure?
Sample questions:
Quiz 3: 1, 11.
<<< It is not
clear how much of the following material will be covered before Quiz 1
>>>
Chapter
2 (pps 26-30)
Energy, temperature and heat.
Kinetic energy - energy of motion. Temperature (which scale?)
provides a measure of the average kinetic energy of the atoms or
molecules in a substance. Heat energy is the total kinetic energy
of all the atoms or molecules in a material. Energy units:
calories.
What is the relationship between energy added to (or removed from) an
object, (delta)E, and the temperature change, (delta)T, that
results? Specific heat or thermal capacity. Water has a
relatively high specific heat.
Temperature scales. Fahrenheit, Celsius, and Kelvin (absolute) scales.
You should know the temperatures of the boiling point of water at sea
level and the melting point of ice on all three scales. The global
average surface temperature of the earth is about what
temperature on the Kelvin scale?
Sample questions: Quiz 1: 4, 10?, 18
Reviews
Mon.
Tue.
Wed.
|
4-5 pm
4-5 pm
4-5 pm
|
Chavez
(Econ) 301
Chavez (Econ) 301
Chavez (Econ) 301
|



