Fri., Jan. 20, 2006
In class
today and during the first part of next Monday's class we will learn
about two more pollutants: tropospheric
ozone and carbon monoxide.
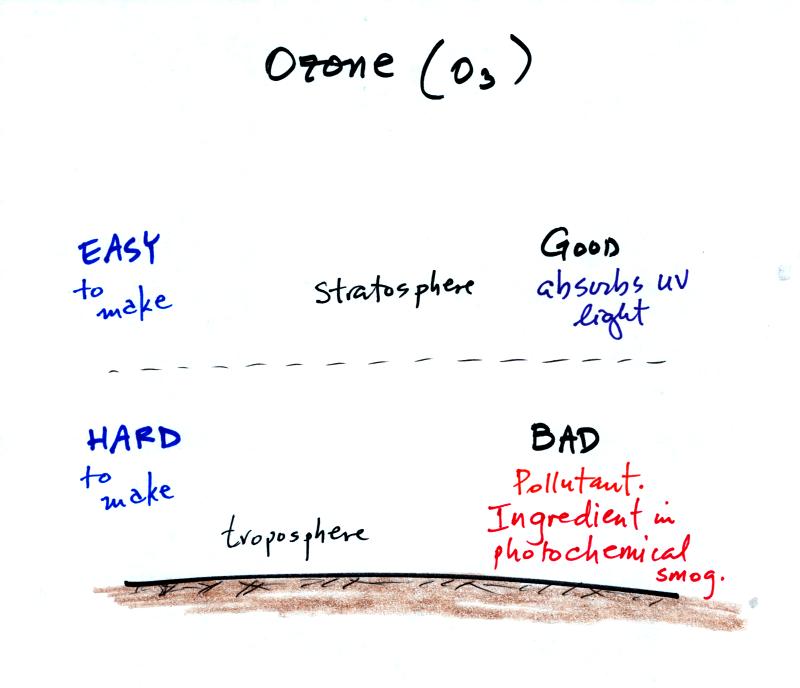
Ozone has a kind of "Dr. Jeckell and Mr Hyde
personality."
Stratospheric ozone is beneficial because it absorbs dangerous
high-energy ultraviolet light. Tropospheric ozone is bad, it is a
pollutant.. It is also a key ingredient in photochemical
smog.
We will try to make some photochemical smog later in a class
demonstration. That will require ozone. We will make use of
the relatively simple stratospheric process for making ozone (see 2
step process below). As we will see a more complex series of
reactions is used in the troposphere.

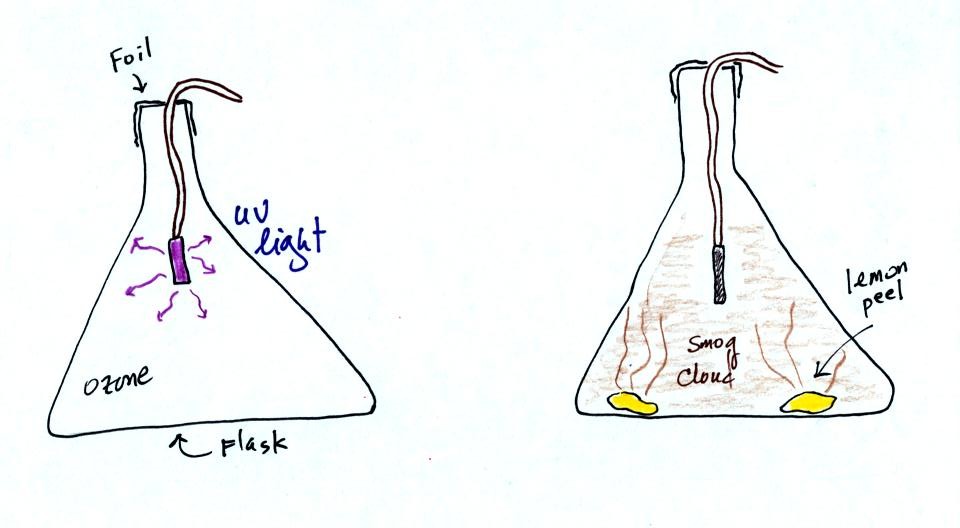
At this
point a small mercury vapor lamp was inserted into a large 4 liter
flask. The lamp emits a lot of (invisible) ultraviolet radiation
and is used
to produce ozone inside the flask. The flask was sealed with foil
so that the ozone couldn't escape. The glass walls of the flask
should absorb the dangerous UV radiation. But just to play it
safe the flask was covered with a black cloth. The ozone will be
used later in the class to make photochemical smog.
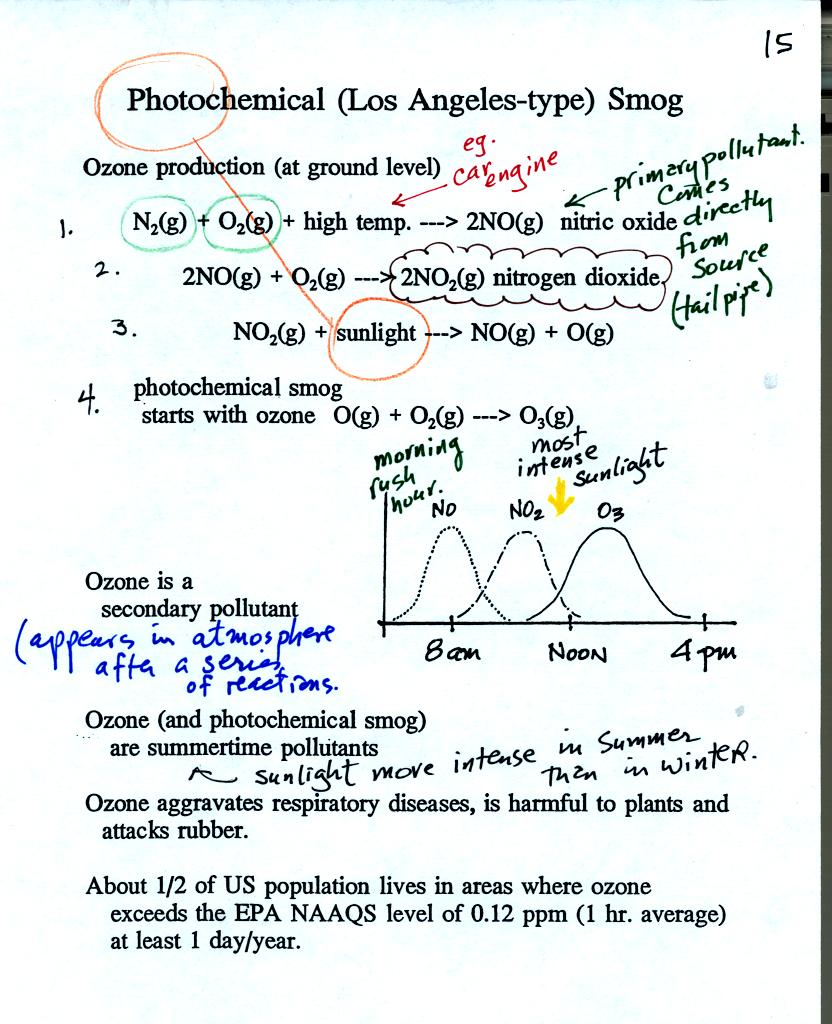
The production of tropospheric ozone begins with nitric
oxide
(NO). NO is produced when nitrogen and oxygen are heated (in an
automobile engine for exampe) and react. The NO can then react
with oxygen to make nitrogen dioxide, a poisonous brown-colored
gas. Sunlight can dissociate (split) the nitrogen dioxide
molecule producing atomic oxygen (O) and NO. O and O2
react (just
as they do in the stratosphere) to make ozone (O3).
Because ozone
does not come directly from an automobile tailpipe or factory chimney,
but only shows up after a series of reactions, it is a secondary
pollutant. The nitric oxide would be an example of a
primary pollutant.
NO is produced early in the day. The concentration of NO2
peaks
somewhat later. Peak ozone concentrations are usually found in
the afternoon. Ozone concentrations are also usually higher in
the summer than in the winter. This is because sunlight plays a
role in ozone production and summer sunlight is more intense than
winter sunlight.
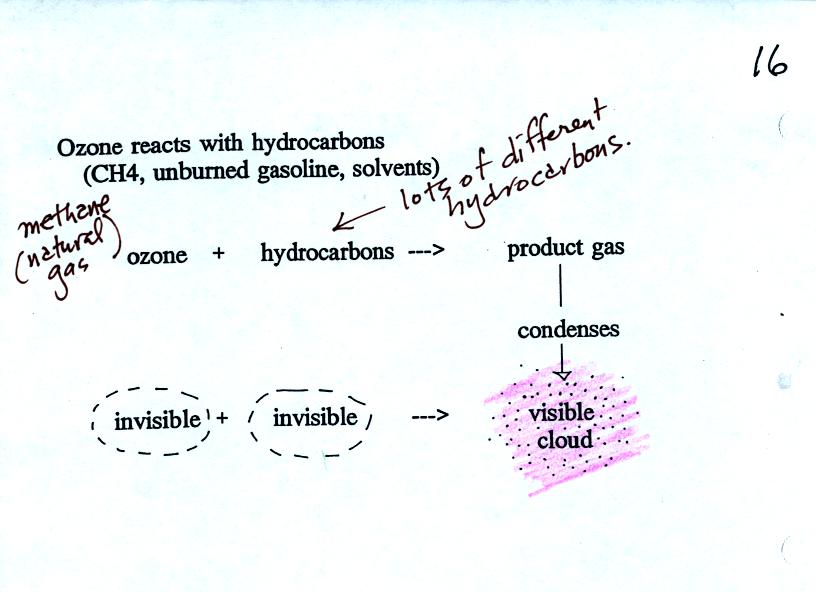
Ozone reacts with a hydrocarbon of some kind to make a
product
gas. This product gas sometimes condenses to make a visible smog
cloud or haze.
The class demonstration of photochemical smog is summarized
below. We begin by using the UV lamp to fill the flask with
ozone. Then a few pieces of fresh lemon peel were added to the
flask. A whitish cloud quickly became visible (colored brown in
the figure below).
We next
briefly discussed the first experiment. With the experiment
materials students instructions concerning the experiment and also the
report they will write about the experiment.

The object of Experiment #2 is to measure the percentage
oxygen
concentration in the air. A moist piece of steel wool is stuck
into a graduated cylinder and the open end of the cylinder is immersed
in a cup of water. Note water won't just enter the cylinder when
you try to immerse it. Air pressure keeps the water out.
You must first insert a piece of flexible tubing into the cylinder
(half inside half outside) and then immerse the cylinder. Lower
it until the water level can just be read on the cylinder scale then
remove the tubing. The experiment is underway.
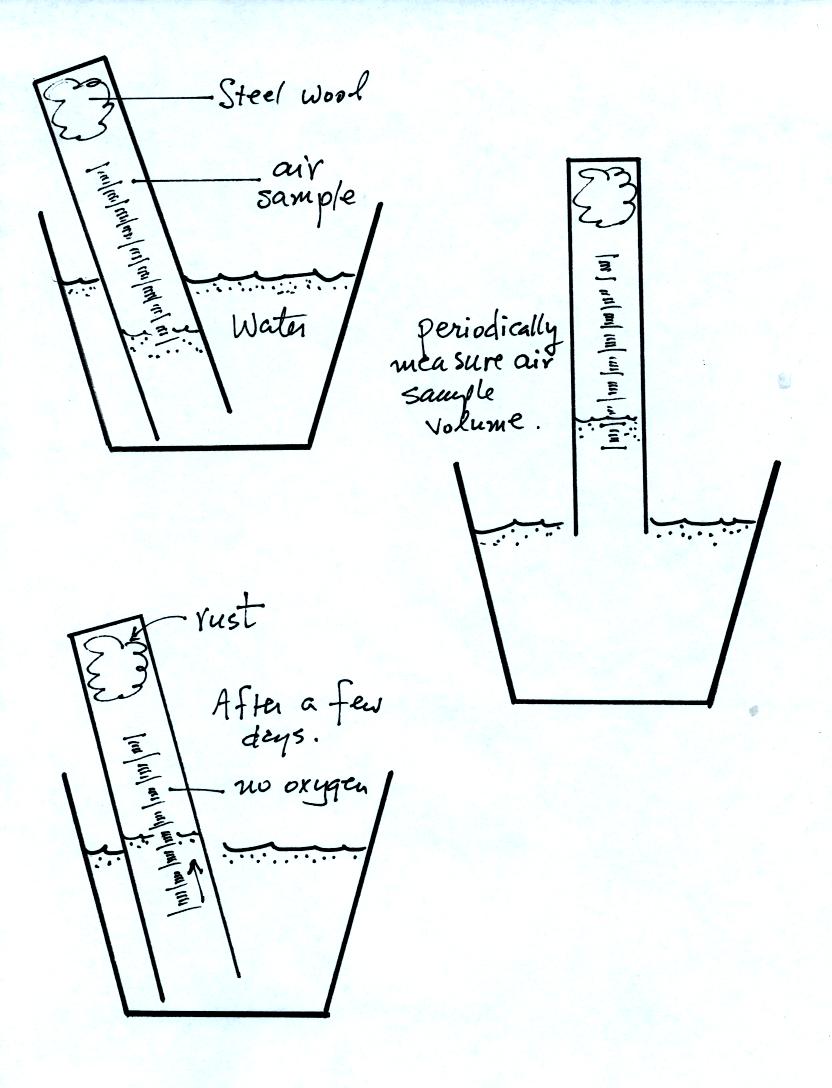
Oxygen in the air inside the cylinder will react with the
steel wool to
make rust. Oxygen is removed from the air sample. As this
occurs the water level will gradually rise (you should explain in your
report why removal of the oxygen causes the water level to
change). Eventually the water level will stop rising, this
indicates that all of the oxygen has been used up and that the
experiment is over.

Carbon monoxide (CO) is a colorless, odorless, toxic gas. It
is a
primary pollutant that results from incomplete combustion (complete
combustion would produce carbon dioxide). The highest CO
concentrations are observed on winter mornings. CO is trapped in
stable morning surface inversion layers.







