Friday Mar. 24, 2006
Mid term grade summaries were distributed in class.
We quickly
reviewed how you can dry out moist air by cooling it to and below the
dew point. This is very much like squeezing on and wringing the
water out of a wet sponge. This was covered
at the end of class on Wednesday.
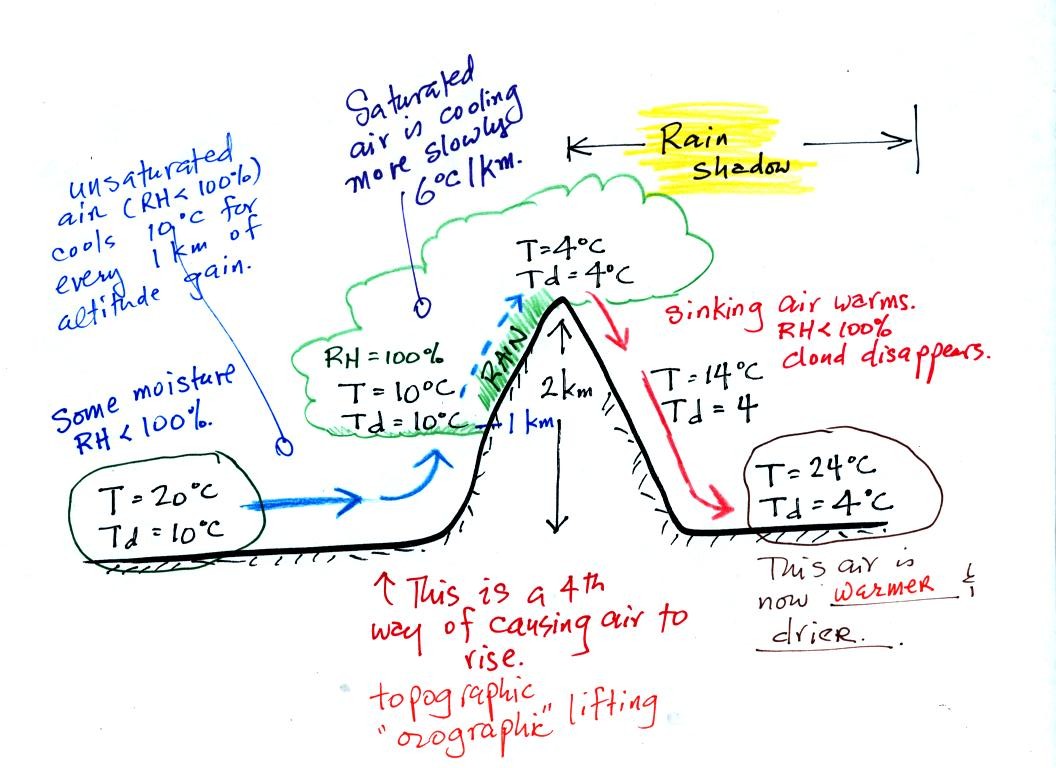
Here's an important cooling and drying out moist air
example. Moist air traveling up and over a mountain cools to its
dew point
temperature at 1 km altitude. A cloud forms at that point.
The parcel of air continues to rise and cool. This additional
cooling below the dew point "squeezes" moisture out of the air.
The moisture falls to the ground as rain. Note that rising
unsaturated air cools at a rate of 10 C/km between 0 and 1 km altitude,
saturated air cools at 6 C/km (release of latent heat in the rising air
during condensation partially offsets the cooling due to expansion)
between 1 and 2 km altitude.
The air travels back down the right side of the mountain and
warms. Note the air ends up warmer (24 C vs 20 C) and drier (Td =
4 C vs Td = 6 C) than when it started out. The downwind side of
the mountain is referred to as a "rain shadow" because rain is less
likely there than on the upwind side of the mountain.
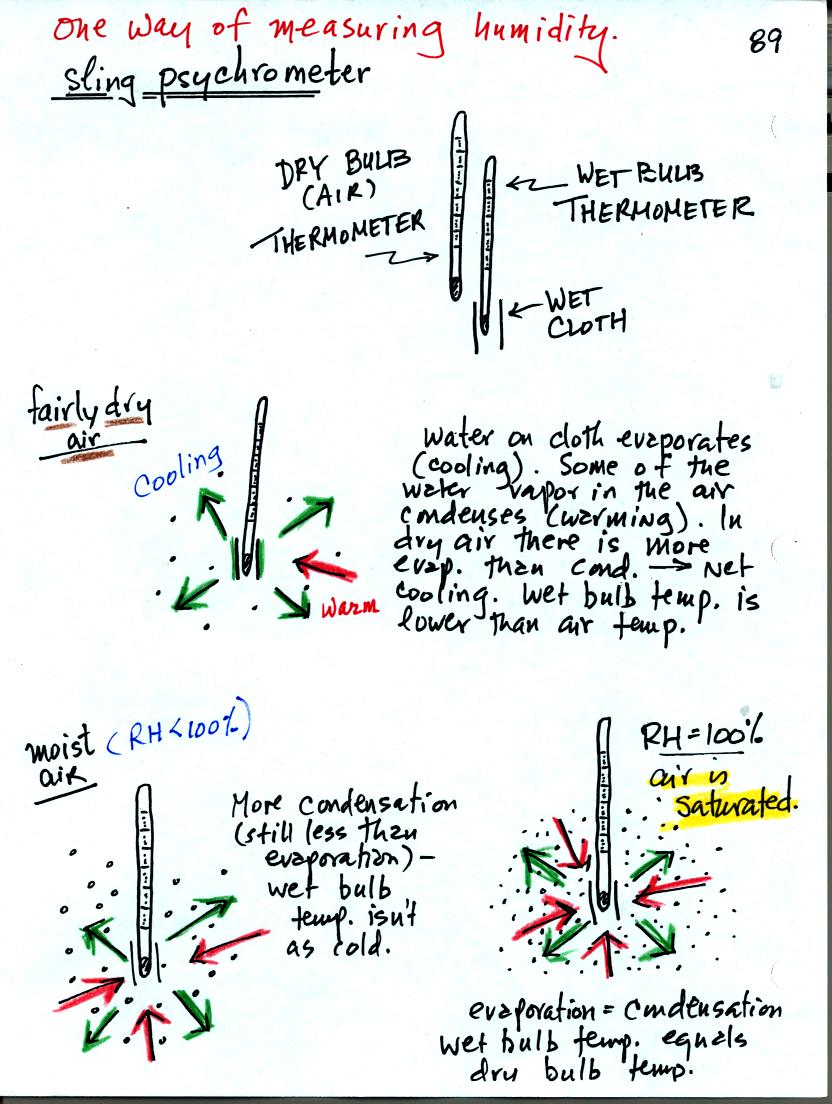
You can use tabulated data (see Appendix D in your textbook) and
measurements made with a sling psychrometer to measure the dew point
and relative humidity. A sling psychrometer consists of two
thermometers mounted side by side. One of the thermometers is
covered with a piece of cloth that is moistened with water. As
the thermometers are swung through the air, water will evaporate from
the wet thermometer. Evaporation is a cooling process. At
the same time some of the water vapor from the surrounding air will
condense onto the thermometer and warm it. The temperature
difference between the two thermometers provides a measure of the
amount of moisture in the air.
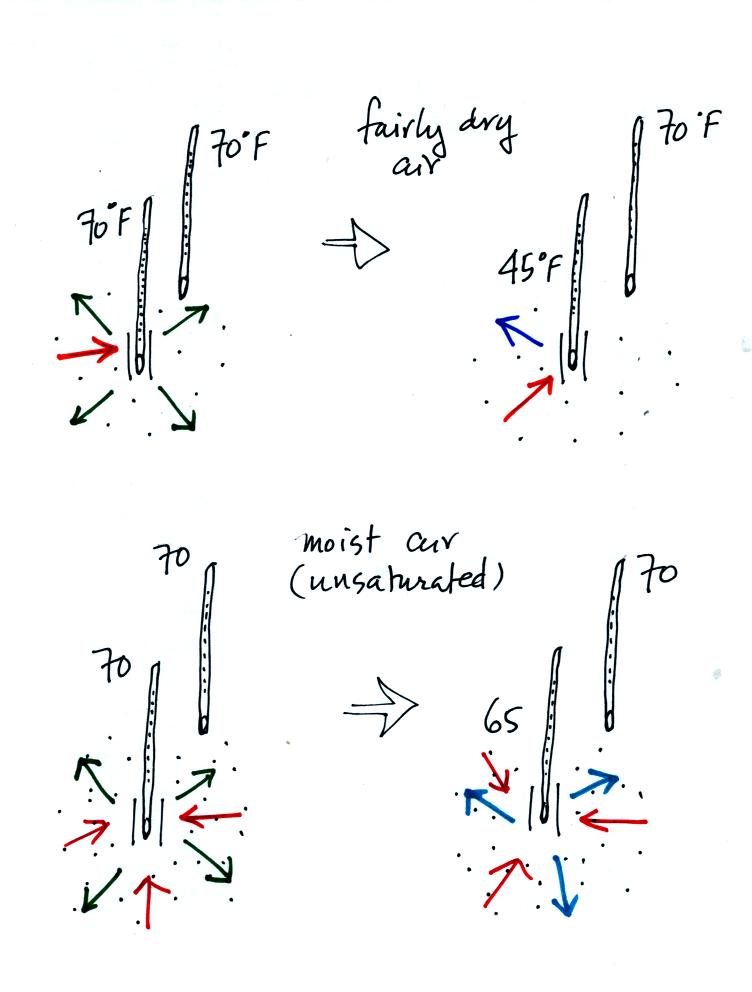
Here's another look at what happens. In the first
case, fairly
dry air, the wet thermometer starts out at 70 F. Because there
are 4
arrows of evaporation and only 1 arrow of condensation the thermometer
cools quickly to 45 F. At 45 F the water on the thermometer
evaporates
at a slower rate. The one arrow of evaporation is balanced by 1
arrow
of condensation. The wet thermometer won't cool below 45 F.
Note the
large, 25 F, difference between the dry and wet bulb thermometers.
In the second case, moister air, the thermometer starts at 70 F again
with 4 arrows of evaporation. Because the air is moister there
are 3
arrows of condensation. The thermometer cools to 65 F. That
lowers
the rate of evaporation slightly. Now the rates of evaporation
and
condensation are equal. With this moister air there isn't as much
of a
difference between the dry and wet bulb thermometer readings.

When you cool air that is next to the ground to the dew point, water
vapor condenses onto objects on the ground such as blades of grass,
your automobile, your morning newspaper, you sleeping cat.
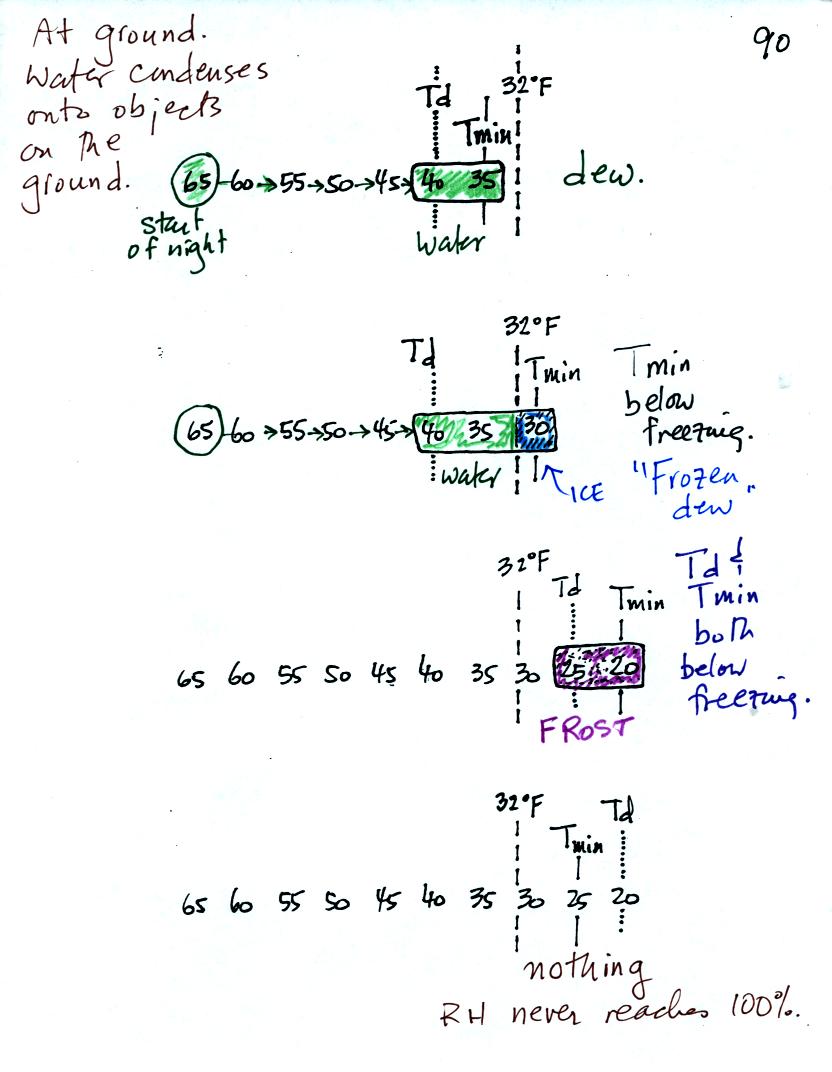
In the first example air that starts out with a temperature
of 65 F
at the beginning of the night. It cools to 35 F during the
night. When the air reaches 40 F, the
dew point, the RH reaches 100%. As the air temperature drops
below the dew point and cools to 35 F water vapor will condense onto
the ground or objects on the ground (such as an automobile). This
is dew.
The dew point is the same but the nighttime minimum temperature
is below freezing in the second example. Dew will form when the
air temperature reaches 40 F. Once the air temperature drops
below 32 F the dew will freeze and form frozen dew.
In the third example both the dew point and nighttime minimum
temperatures are below freezing. When the air temperature drops
below the dew point, water vapor turns directly to ice (deposition) and
forms frost.
The dew point in this case is sometimes called the frost point.
The air never becomes saturated in the fourth example because the
nighttime minimum temperature never cools to the dew point.


When air above the ground becomes saturated the water vapor
condenses
onto small particles in the air called cloud condensation nuclei
(CCN). With some hygroscopic materials condensation begins when
the RH
is less than 100% (see p. 92, reproduced at the end of today's notes,
for more explanation of why this occurs).
A short video showing how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of glass.

The start of the video at left showed the small grains of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt (shown above at right).

This is an example of a midterm grade summary. The first
two lines show the quiz scores and percentage grades. The third
line is the total optional assignment extra credit points that have
been earned. The report grade and total 1S1P points are shown on
the 4th line. A 0 for the expt/book report grade means your
report hasn't yet been graded yet or you haven't turned in a
report. If you haven't completed a report or aren't now working
on a report you should check with the instructor immediately.
Please check to be sure all of your scores haven't been recorded
correctly. You'll have to trust that the writing percentage
grade and the overall averages have been calculated correctly.
Note it is the first of the overall average scores (no quiz scores
dropped) that must be 90.0 or above in order to not have to take the
final exam. Otherwise it is the second grade (lowest quiz
dropped) that is of interest.
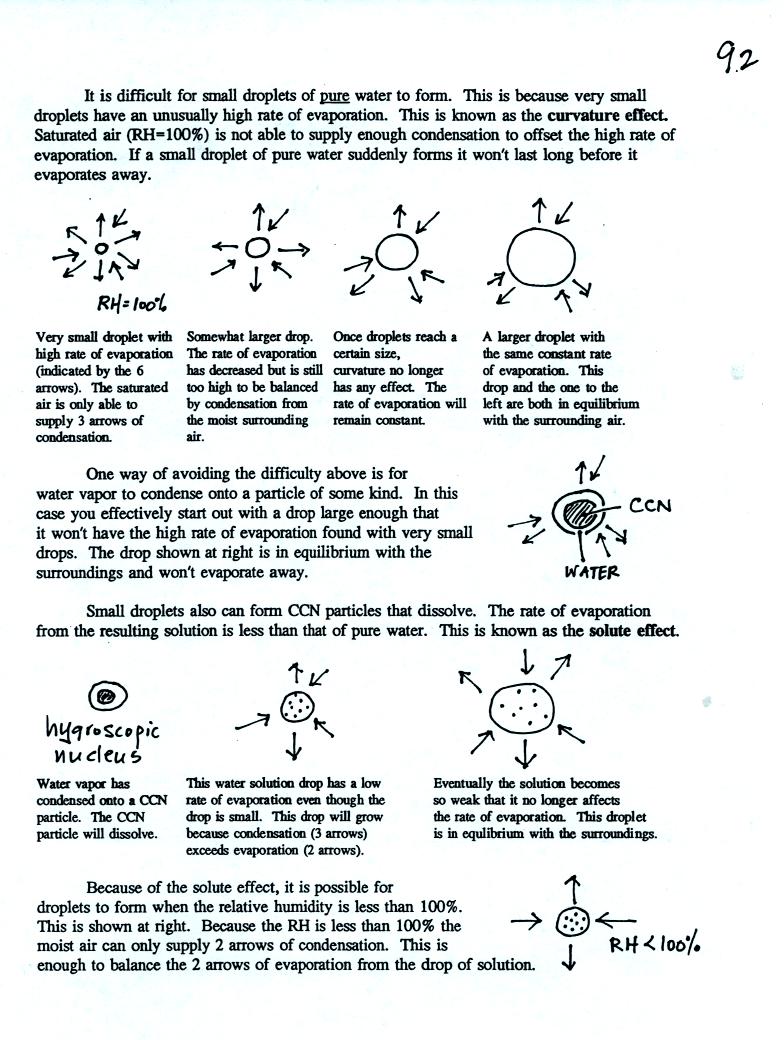
Here's
some more information about the formation of cloud droplets.
This wasn't covered in class and you do not need to worry about
learning about these details. This is one of those "for further
reading" sections.