Tue., Apr. 4, 2006
The revised Expt. 2 reports (and any other reports turned in last week)
have been graded.
There have been a few changes to the Quiz 3
Study Guide. A printed version of the study guide was
distributed in class.
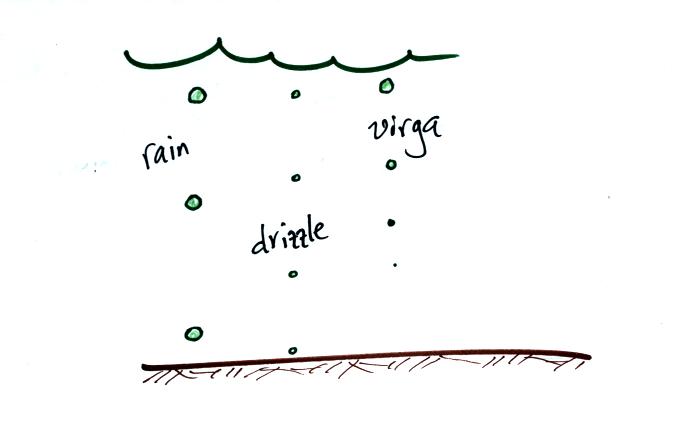
The collision coalescence process only produces rain, drizzle, and
virga. That's not much variety.
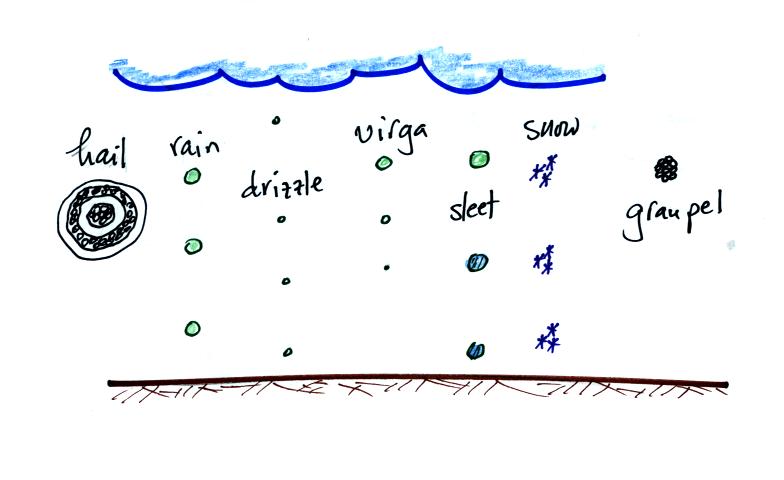
Look at what the ice-crystal process can do. It should be
said, however,
that some of these different types of particles form between the cloud
base and the ground.
The ice crystal process works in cold clouds, clouds that contain ice.
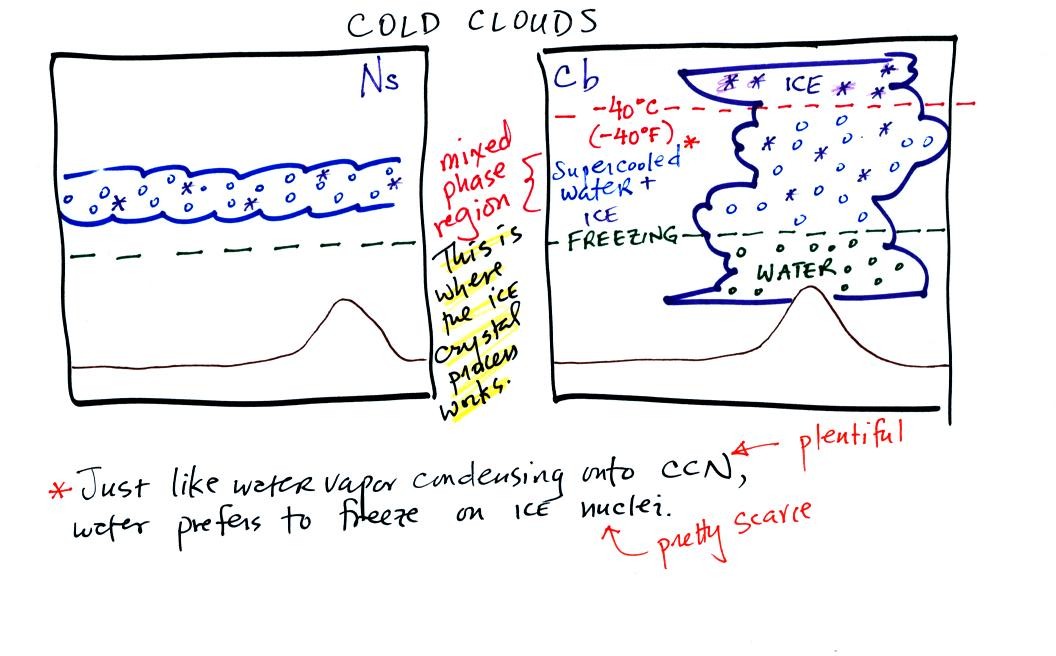
A large part of thunderstorm clouds and all of nimbostratus
clouds
are composed of a mixture of supercooled water droplets (water that has
been cooled to below freezing but hasn't froze) and ice crystals.
This is called the mixed phase
region. This is where the ice crystal process will produce
precipitation. This is also where the electrical charge that
results in lightning is generated.
In this mixture of ice crystals and supercooled water droplets, the ice
crystals are able to grow even when the water droplets do not.
This is explained in the next series of three figures (see p. 101 in
the photocopied notes).
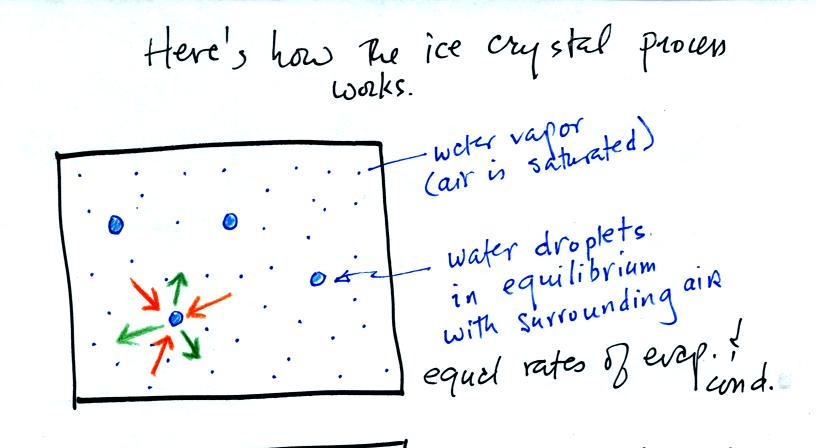
The supercooled water droplets are in equilibrium with their
surroundings. Otherwise the cloud wouldn't be there, it would
evaporate away. This means the surrounding air must be saturated
(RH=100%) in order to supply enough condensation to balance out
evaporation from the droplets. The little "specks" in the picture
represent water vapor. Note the higher water vapor concentration
above compared to the picture below.
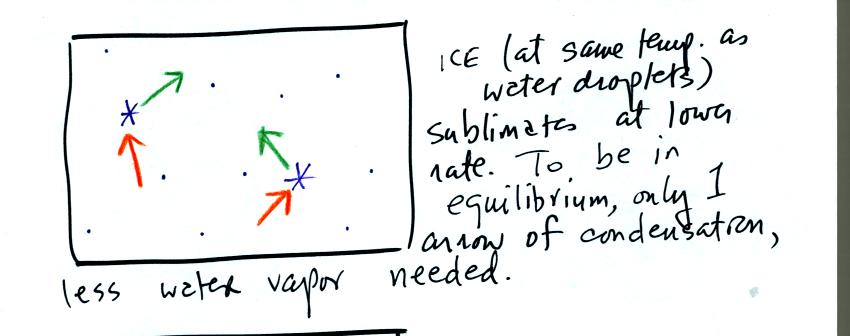
It takes more energy to break bonds in an ice crystal and turn
into water vapor than to change from water to water vapor. An ice
crystal at the same temperature as a water droplet won't sublimate away
as quickly as a water droplet evaporates. Less moisture is
required in the surrounding air to keep an ice crystal in equilibrium.
This is illustrated in the following analogy
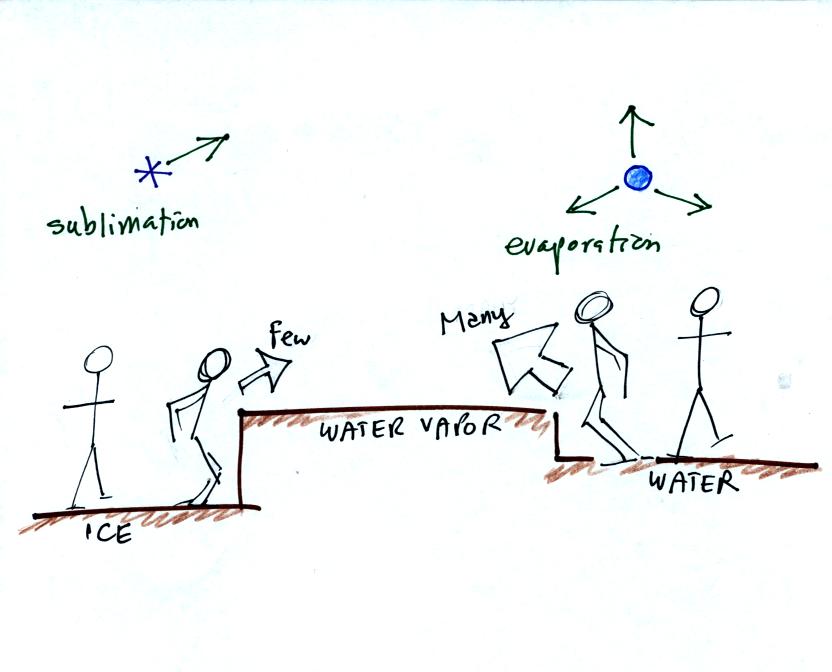
Fewer people are able to make the 2 or 3 foot jump than
people able to
make the 1 foot jump.

The ice crystals are found in the moist environment required
by the water droplets. More water vapor is being deposited
onto the ice crystal than it is is losing
by sublimation. The ice crystal will grow, the water droplets are
in equilibrium and won't change size.
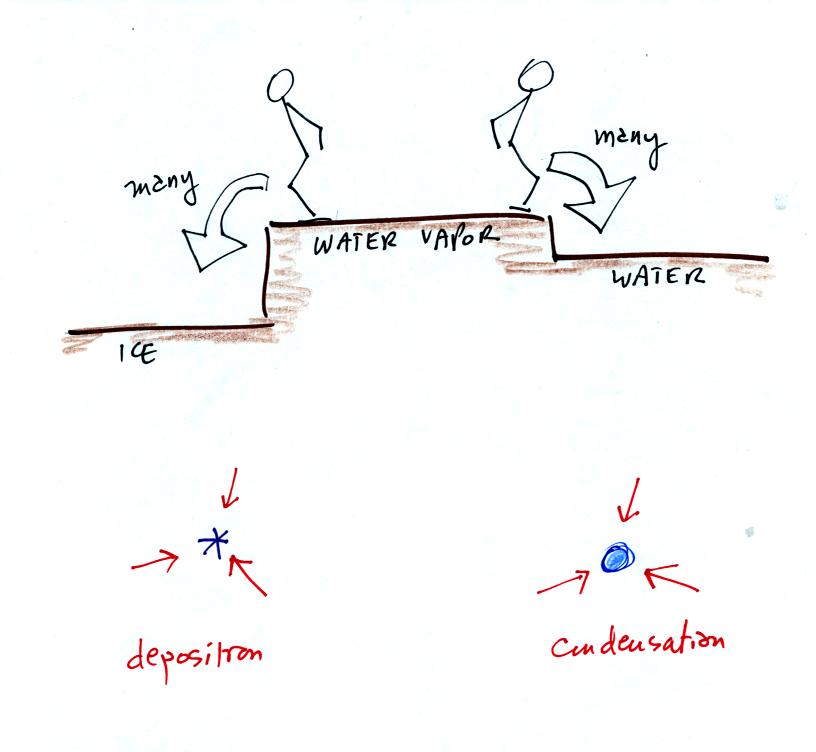
The rates of deposition and condensation depend on the
amount of
water vapor surrounding the ice crystal and water droplet. Since the
same amount of water surrounds both particles, the rates of deposition
and condensation will be equal.

Once an ice crystal has grown a little bit it becomes a snow
crystal. Snow crystals can have a variety of shapes (called
crystal habits, sketched above) depending on the conditions
(temperature and moisture) in the cloud. Dendrites are the most
common because they form where there is the most moisture available for
growth. With more raw material available it makes sense there
would be more of this particular snow crystal shape.
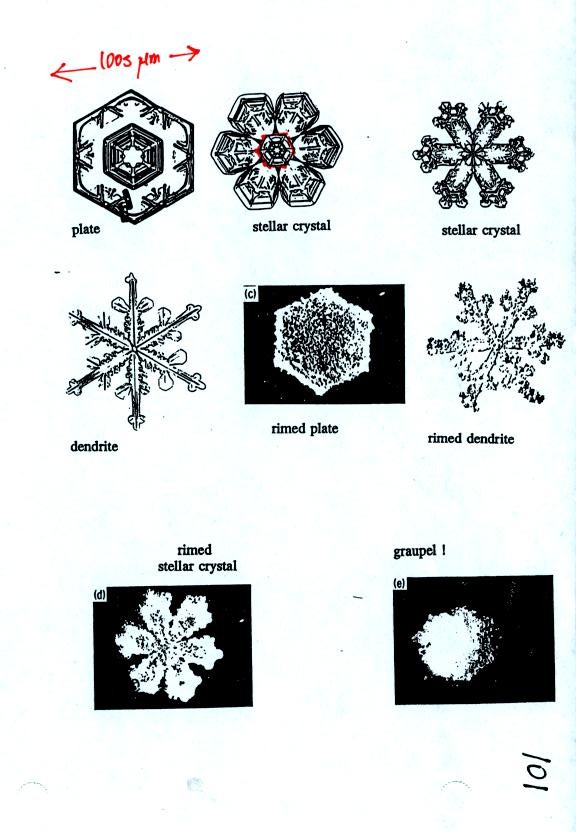
Some actual photographs of snow crystals (taken with a
microscope). You'll find even better photographs at snow
crystal pictures
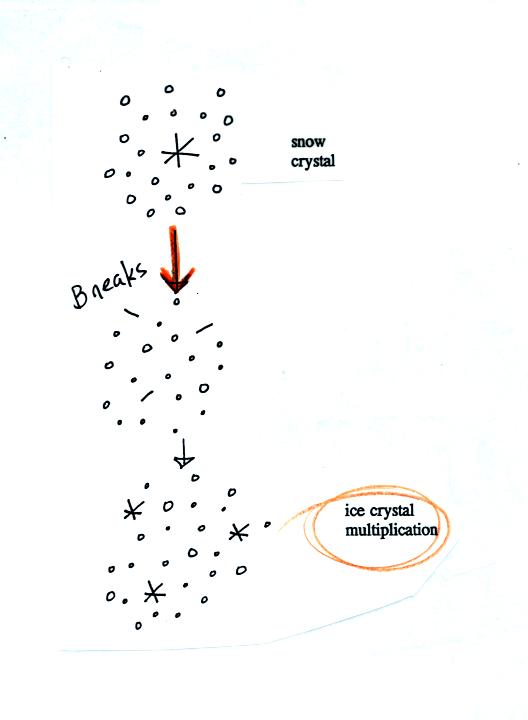
A variety of things can happen once a snow crystal
forms. First
it can break into pieces, then each of the pieces can grow into a new
snow crystal. Because snow crystals are otherwise in rather short
supply, ice
crystal multiplication is a way of increasing the amount of
precipitation that ultimately
falls from the cloud.
This is incidentally the idea behind cloud seeding, to increase the
number of ice crystals and hopefully the amount of precipitation.
A substance called silver iodide is often used. Silver iodide is
one of the relatively rare materials that can act as an ice crystal
nucleus. However it is possible to "overseed" a cloud and end up
with too many ice crystals. Then they all fight for a limited
amount of water vapor and, as a result, do not get very big.
Overseeding a cloud could decrease the precipitation from a cloud.
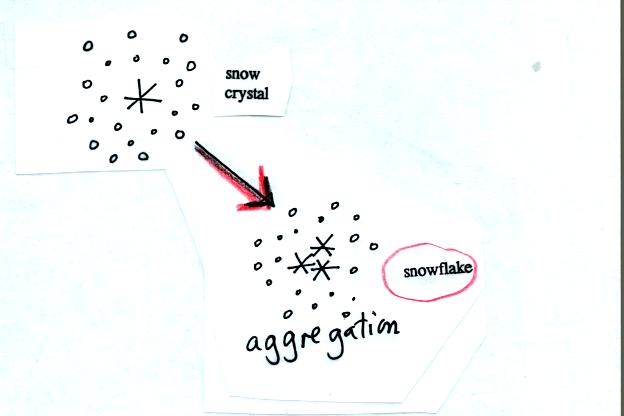
Several snow crystals can collide and stick together to form
a
snowflake. Snow crystals are small, a few tenths of a millimeter
across. Snowflakes can be much larger and are made up of many
snow crystals stuck together. The sticking together or clumping
together of snow
crystals is called aggregation.
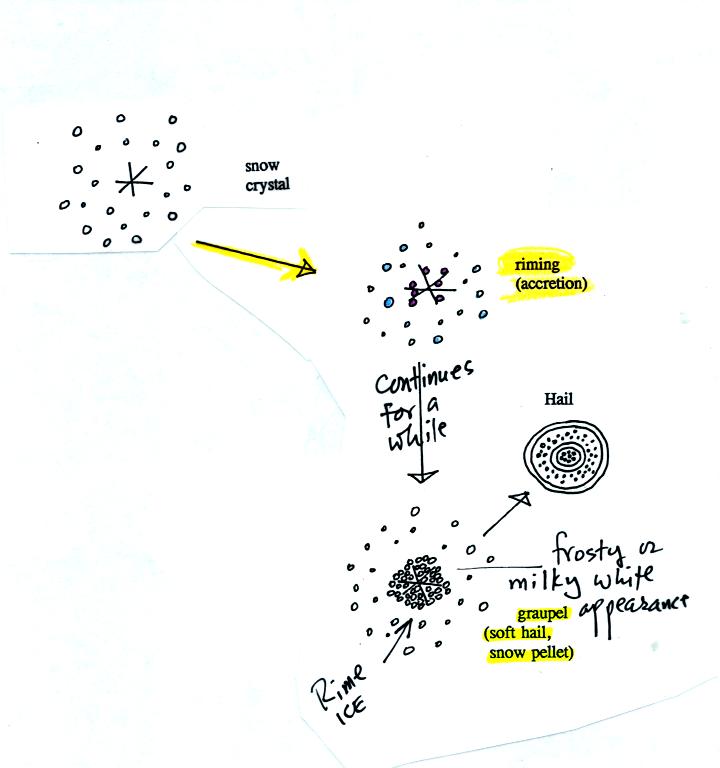
Snow crystals can collide with supercooled water
droplets. The
water droplets may stick and freeze to the snow crystal. This
process is called riming or accretion (note it is really the same idea
as collision and coalescence). If a snow crystal collides with
enough water droplets it can be completely covered with ice. The
resulting particle is called graupel (or snow pellets). Graupel
is sometimes mistaken for hail and is called soft hail. Rime ice
has a frosty milky white appearance. A graupel particle resembles
a miniature snow ball. Graupel particles often serve as the
nucleus for a hailstone.
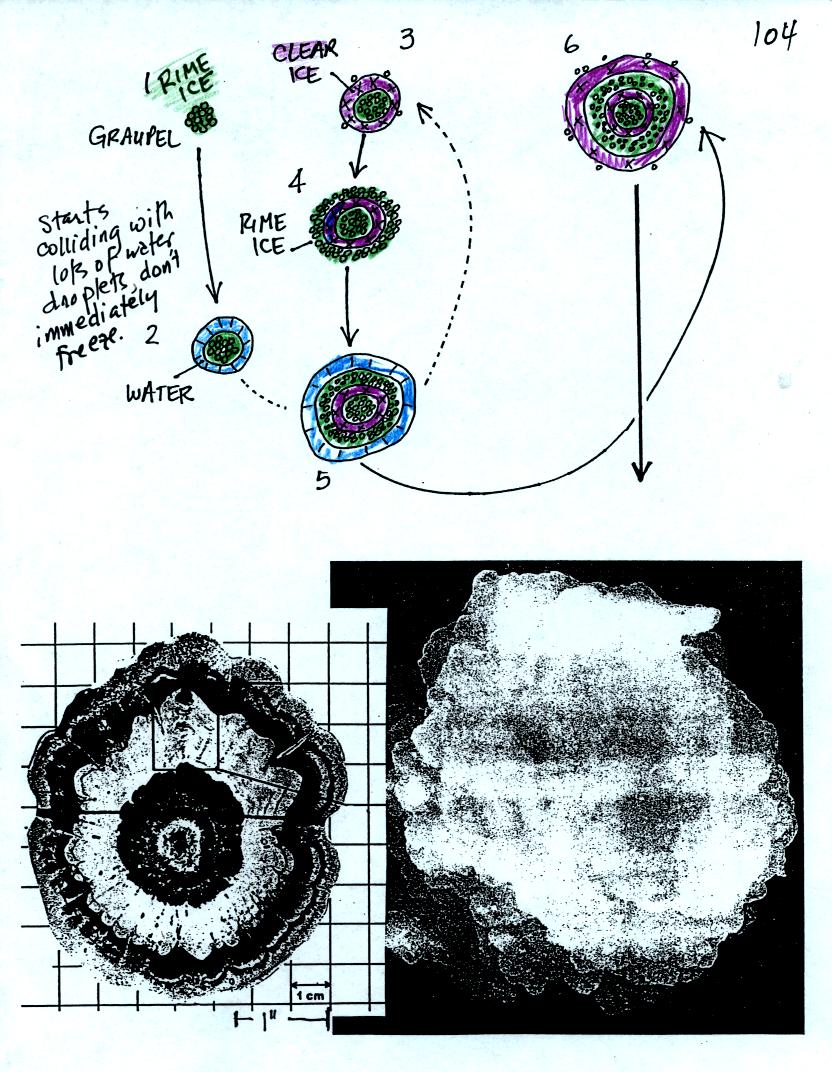
Hail forms in thunderstorms with very strong updrafts.
In the
figure above the hailstone starts with a graupel particle (colored
green to represent rime ice). The graupel falls or gets carried
into a part of the cloud where it collides with a large number of
supercooled water droplets which stick to the graupel but don't
immediately freeze. The graupel gets coated with a layer of
water (blue). The particle then moves into a colder part of the
cloud
and the water layer freeze producing a layer of clear ice (the clear
ice, colored violet, has a distinctly different appearance from the
milky white rime
ice). The particle then can pick up a new layer of rime ice,
followed by another layer of water which subsequently freezes to
produce a layer of clear ice.
Large hailstones can be composed of many alternating layers of rime and
clear ice. An unusually large hailstone (2.5 to 3 inches in
diameter) has been cut in
half to shown the different layers of ice.
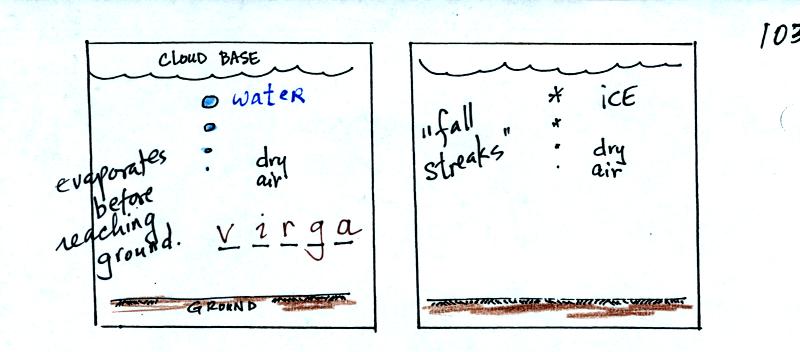
The ice crystal process can produce a variety of types of
precipitation
particles inside the cloud. Once the precipitation particle falls
from the cloud it change in a variety of ways before it reaches and
hits the ground.
In the example above at left the particle first melts and then
evaporates before reaching the ground. Rain that evaporates
before reaching the ground is called virga. A similar thing can
happen with snow crystals or snow flakes. They sublimate away;
the streamers of falling precipitation are called fall streaks.
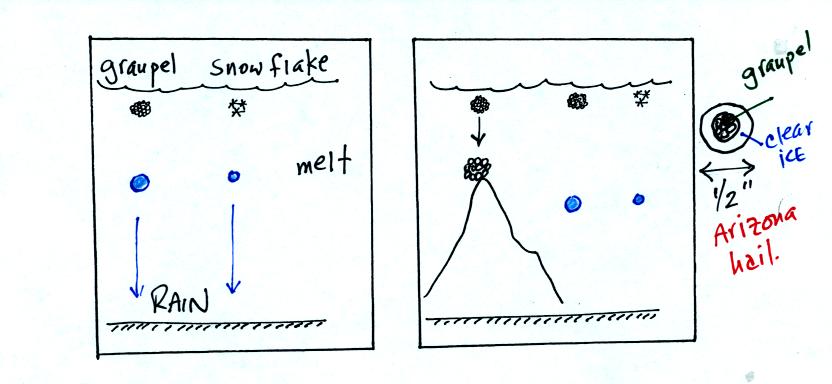
The frozen precipitation particles produced by the ice
crystal process
(graupel or snow) can melt before reaching the ground. This would
be rain (or drizzle if the drops are small). Rain in most
locations at most times of the year starts out as frozen precipitation..
If you are on a mountain top you might see some of the frozen
precipitation before it melts. You might see graupel falling from
a summer thunderstorm, for example, while the people in the valley only
observe rain.
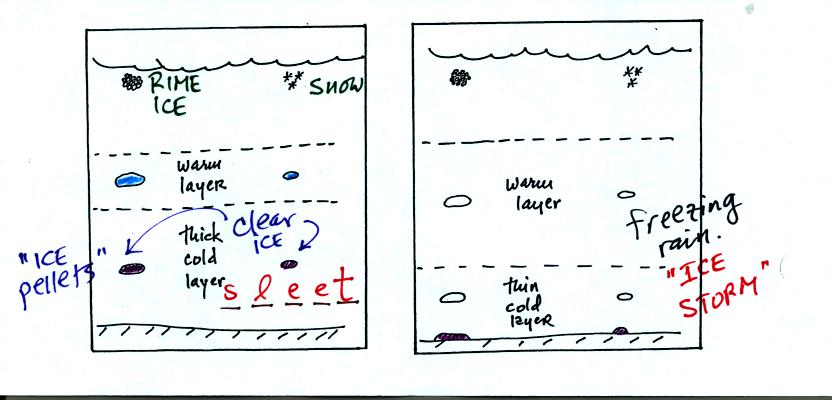
Sometimes the frozen precipitation will melt and then fall
into a thick
layer of cold air and refreeze. The resulting particle is called
sleet (or ice pellets). The clear ice in sleet is noticeably
different from the rime ice in graupel.
Rain that falls into a shallow cold air layer and freezes after
reaching the ground is called freezing rain. It is nearly
impossible to drive during one of these "ice storms." Sometimes
the coating of ice is heavy enough that power lines are brought down
and branches on trees are broken.
Now we
move onto a different topic to finish the class - satellite photographs
(see pps 99-100 in the photocopied notes)
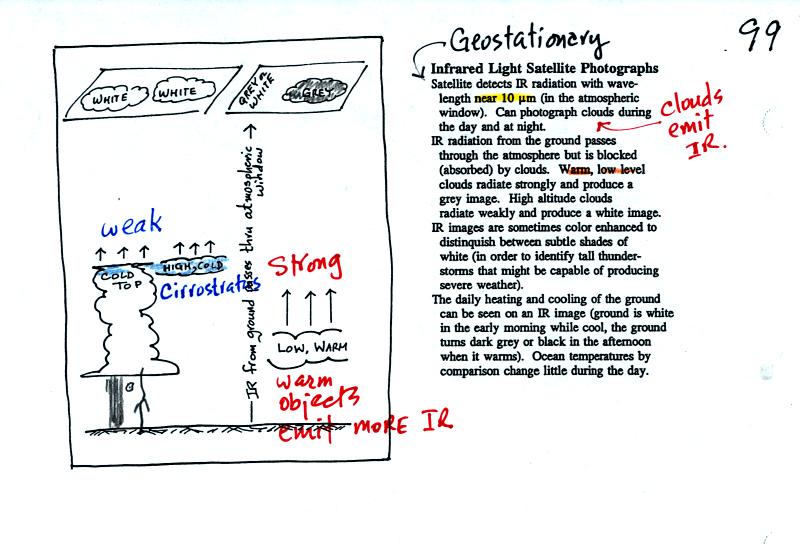
An infrared satellite photograph detects the IR radiation
actually
emitted by clouds. You don't depend on seeing reflected
sunlight. Clouds can be photographed during the day and at
night. White on an IR photograph means the top radiating surface
of the cloud is cold (found at high altitude). Warm, low level
clouds appear grey.

Thick clouds produce a white image on a visible satellite
photograph. Thin clouds appear grey. Note a thunderstorm
appears white on both IR and VIS satellite photographs.
The origin of the patchy cloud pattern seen behing cold fronts that are
out over the ocean is shown below.

Cold air warms and is moistened as it passes over warmer
ocean
water. The air can eventually become bouyant and rise enough that
a cloud forms. This is essentially the same as the Lake Effect
discussed on p. 204 in Ch. 8 of the text.
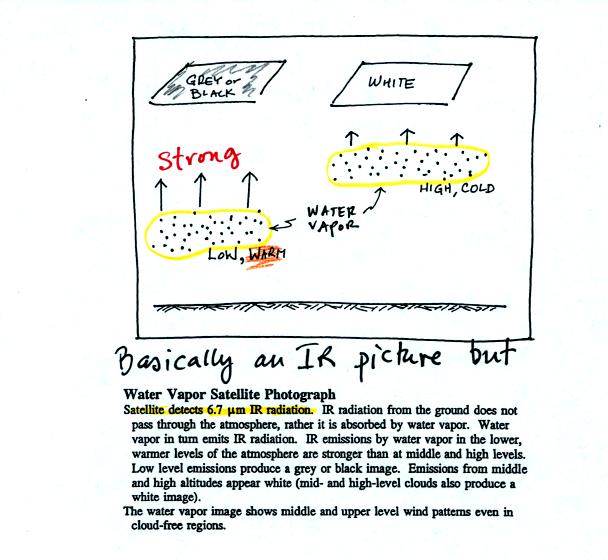
Finally a water vapor satellite photograph is similar to an
IR
photo. In this case it is water vapor, not clouds, that emit IR
radiation (at a slightly different wavelength) that is detected and
displayed by the satellite. Water vapor found at low altitude is
warm and appears grey on the photograph. High altitude water
vapor is cold and appears white.




















