Tues., Jan. 24, 2006
The 1S1P Assignment #1 reports are due one week from today.
The Experiment #1 reports are due in two weeks on Tue., Feb. 7.
You should return your materials well before that date so that you can
pick up the Supplementary Information sheet for Expt. 1.
The first of the semester's homework assignments will be distributed in
class on Thursday and will be due a week from Thursday. You can
earn a small amount of extra credit by doing these optional
assignments.
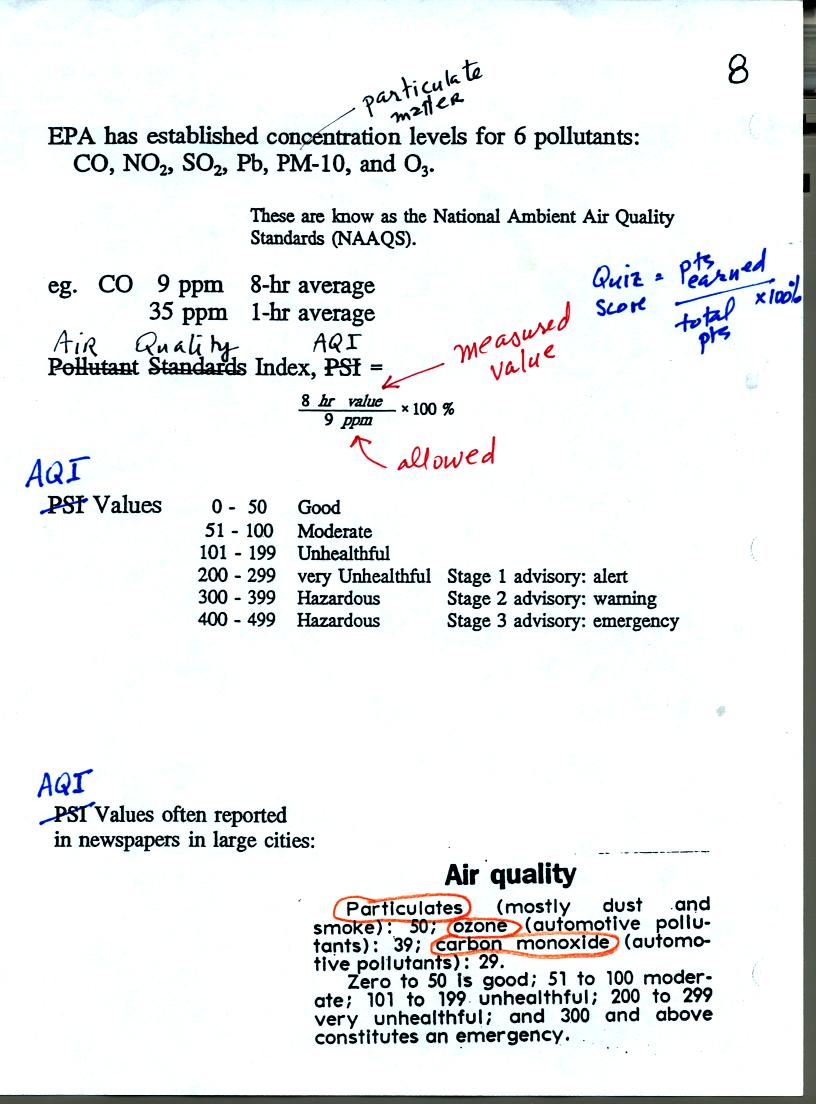
Concentrations of several pollutants are measured daily in
many
cities (particulate matter, ozone, and carbon monoxide are monitored in
Tucson) and measured values are reported in the newspaper or on
television using the Air Quality Index (formerly the pollutant
standards index). This is basically the measured value divided by
the allowed value multiplied by 100%. Current Air Quality Index values for
Tucson are available online.
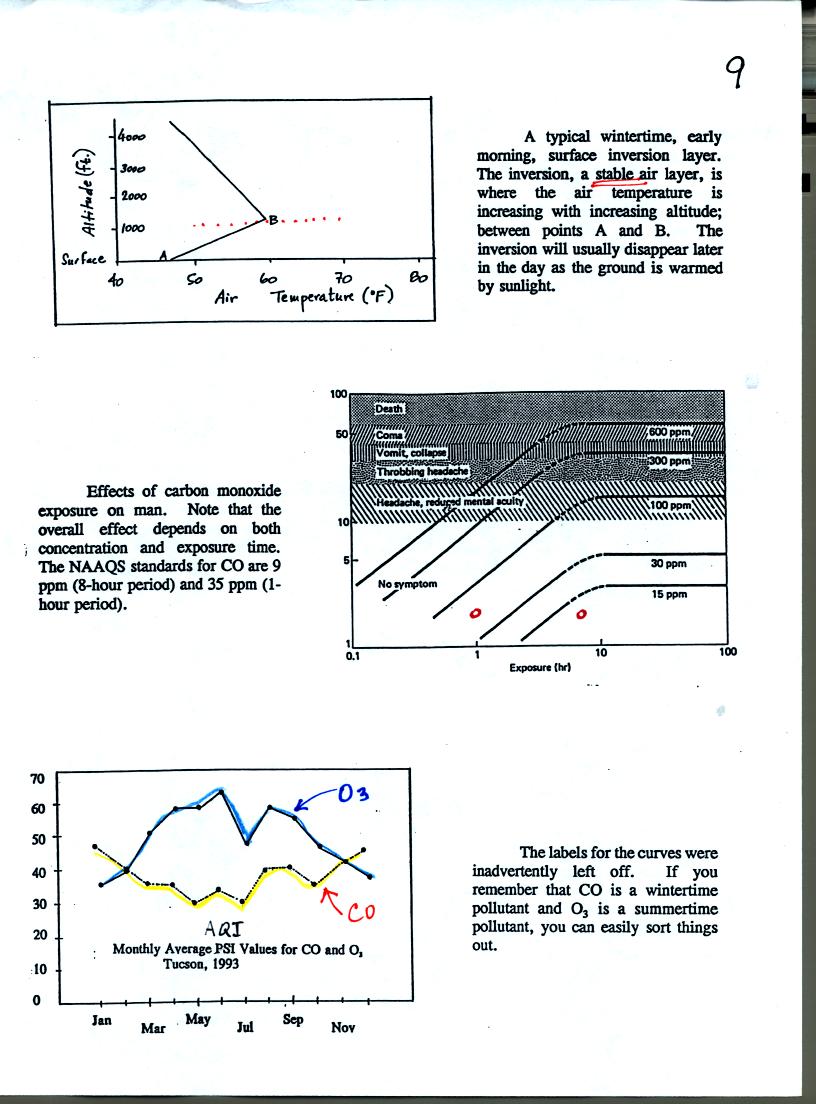
This ends are coverage of air pollutants for this
semester. You
can find more information about air pollutants at the Pima County Department of
Environmental Quality web page.
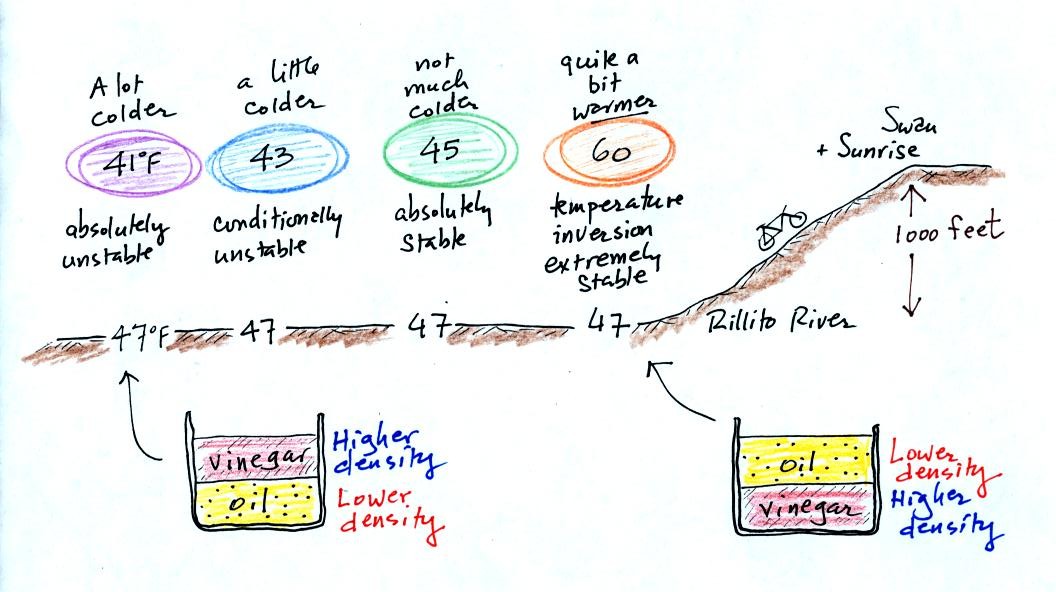
The rate at which air temperature decreases with increasing
altitude determines whether the atmosphere is stable or unstable.
4 different scenarios are shown above. When the air cools
as rapidly as it does in the left most example above (6o F/ 1000 ft.)
the atmosphere is absolutely unstable. This is somewhat analogous
to trying to put some vegetable oil on the bottom of a glass and then
pouring vinegar above it. The oil is less dense and will float on
the water in the vinegar. The oil and the vinegar in the glass
would quickly trade places. Thunderstorm formation requires
unstable atmospheric conditions.
The middle example shows conditional instability (we'll see what the
conditions are later in the semester).
A temperature inversion like shown at right in the figure above is a
fairly common occurrence on winter mornings in Tucson. This
produces extremely stable atmospheric conditions. Air next to the
ground will not freely mix with air overhead. Pollutants released
into the air layer at the ground will increase in concentration because
they cannot mix with and be diluted by cleaner air above.
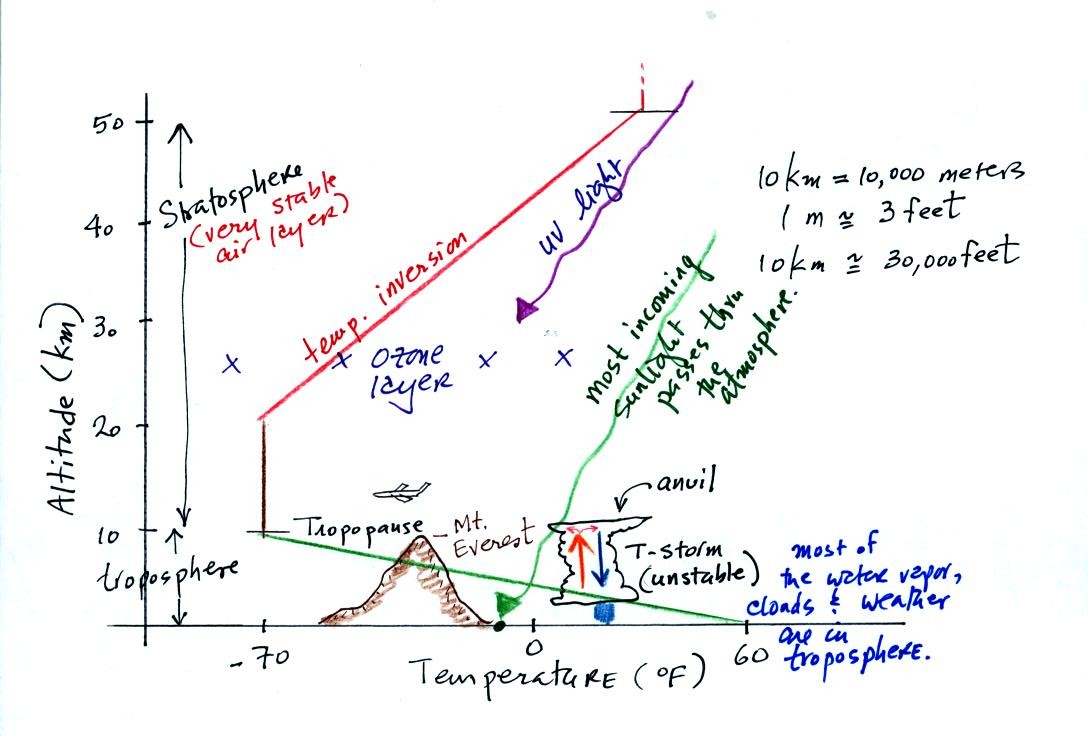
The atmosphere can be split into layers
depending on whether
temperature is increasing or decreasing with increasing altitude.
The two lowest layers are shown in the figure above. We live in
the troposphere. The troposphere is found, on average, between 0
and about 10 km altitude, and is where temperature using decreases with
increasing altitude. Most of the sunlight arriving at the top of
the atmosphere passes through the atmosphere and is absorbed at the
ground. This warms the ground. The air in contact with the
ground is warmer than air higher up and further from the ground.
The troposphere contains most of the water vapor
in the atmosphere and is where most of the weather occurs. The
troposphere can be stable or unstable. The thunderstorm shown in
the figure indicates unstable conditions, meaning that strong up and
down air motions are possible. When the thunderstorm reaches the
top of the troposphere, it runs into the stable stratosphere. The
air can't continue to rise in the stable stratosphere so the cloud
flattens out and forms an anvil.
At nearly 30,000 feet altitude, the summit of Mt.
Everest is near the top of the troposphere. Commercial aircraft
fly at cruising altitudes between 30,000 and 40,000 feet. This is
right at the boundary between the top of the troposphere and the bottom
of the stratosphere.
The ozone layer is found in the stratosphere. Absorption of
ultraviolet light by ozone warms the air in the stratosphere and
explains why temperature increases with increasing altitude between 20
and 50 km altitude.
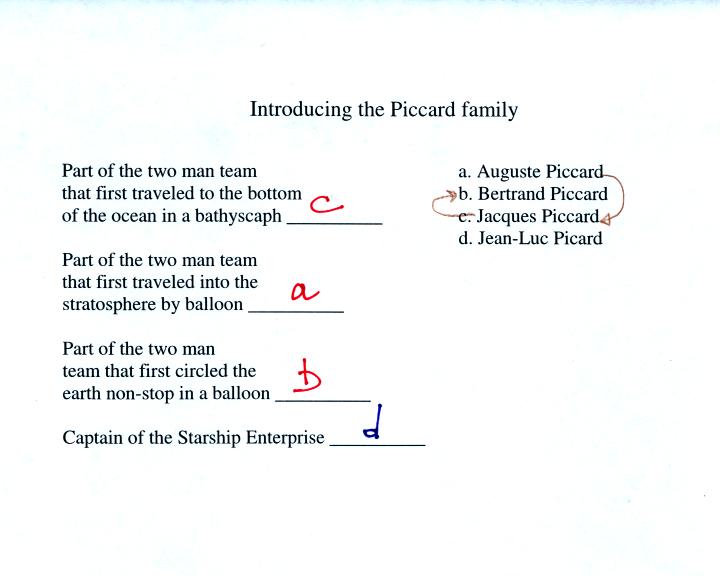
In the the next portion of class we watched a short segment
from a
PBS program titled "The Adventurers." This episode covered the
first manned balloon flight into the stratosphere (August Piccard and
Paul Kipfer). This and other manned balloon flights are
summarized
on pps 31 and 32 in the photocopied class notes.


Next we will start trying to understand air pressure. Air
pressure is important because small differences in air pressure can
start the wind blowing (sometimes violently). Before tackling
pressure we will review mass, weight, and density.
Mass is just the amount of material in an object. A Cadillac has
more mass than a Volkswagen. The Cadillac has more raw material
than a VW bug.
Gravity acting on a mass produces weight. Weight is a force, mass
is not a force. We sometimes use mass and weight
interchangeably. We can do this because gravity of the earth
doesn't change.
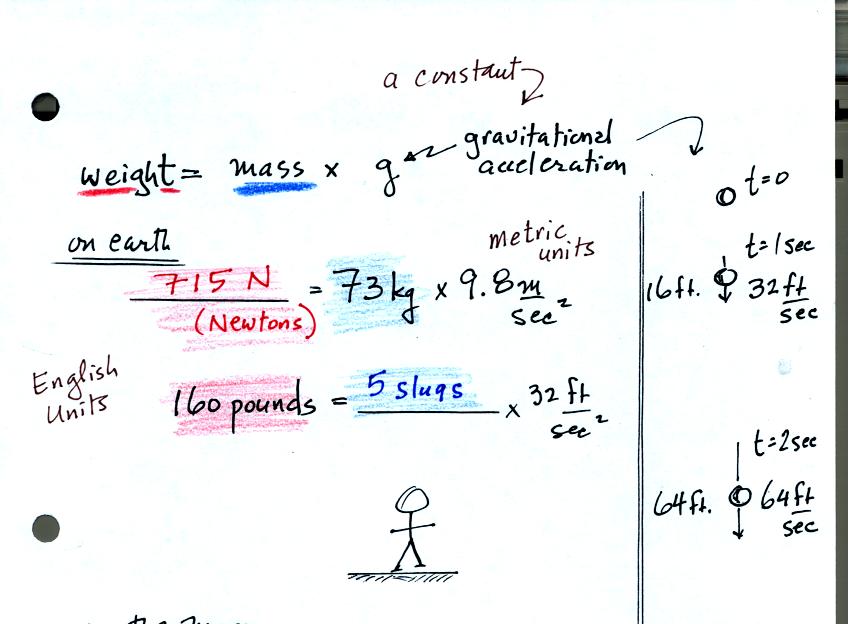
Weight is just mass multiplied by a constant called the gravitational
acceleration. On the earth g is 9.8 m/sec2 or 32 ft/sec2.
The gravitation acceleration depends on the size (radius) and mass of
the earth.
You've probably heard of kilograms (kg) and pounds (lbs).
Kilograms are units of mass
in the metric system, pounds are English system units of weight. It is OK to
use them interchangeably on the earth because a mass of 73 kg will
always produce a weight of 160 pounds. Strictly speaking the
metric system units of weight are units (the 73 kg mass has a weight of
715 Newtons). The units of mass in the English system are
slugs. A 5 slug object (73 kg in the metric system) weights 160
pounds.
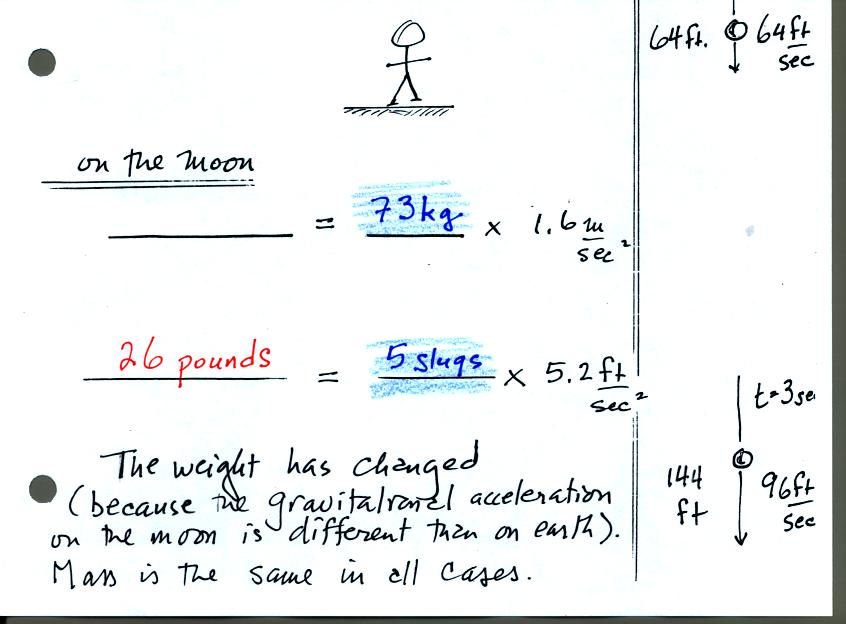
On the earth a person with a mass of 73 kg weighs 160 pounds. If
we travel to the moon however gravity will be different. On moon
the person will still have a mass of 73 kg or 5 slugs. The
person's weight however will be different because the value of the
gravitational constant will be different than on the earth (the moon is
smaller and has less mass than the earth). On the moon, a person
with a mass of 73 kg will only weigh 26 pounds.
Bottles
containing equal volumes of water and mercury were passed around the
classroom. The mercury was much heavier, it had more mass than
the water. What explains this difference in mass?
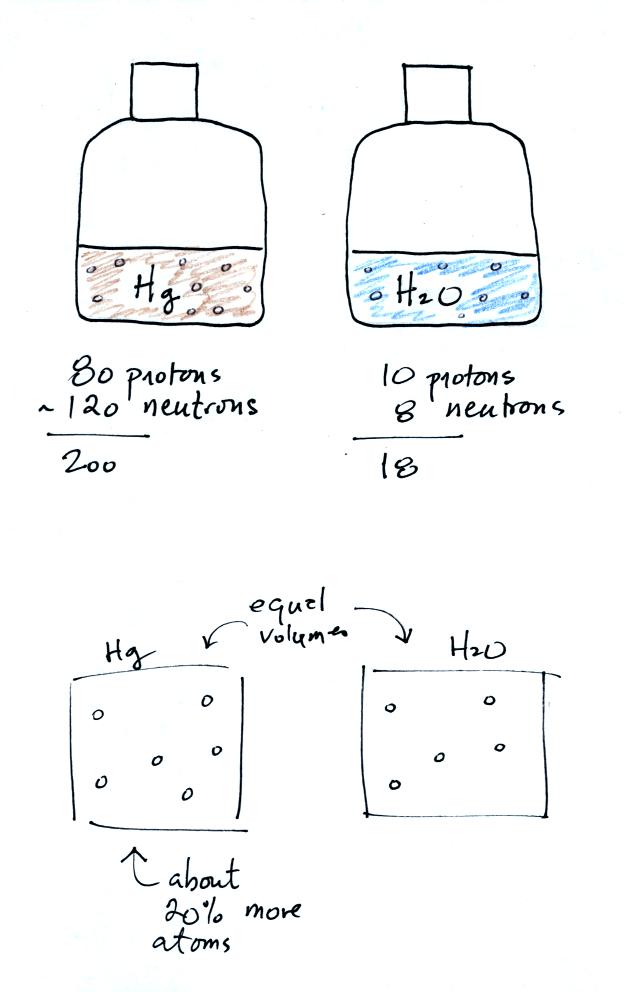
Mercury has a density of 13.6 g/cm3, water a density of 1 g/cm3.
There is 13.6 more mass in the volume of mercury than in the same
volume of water.
The main reason for the difference is that a mercury atom has more
protons and neutrons than a water molecule.
Mercury has an atomic number of 80 which means there are 80 protons in
its nucleus. Mercury's atomic weight is about 200; mercury has 80
protons and about 120 neutrons (mercury comes in slightly different
forms or isotopes: some atoms have 121 or 122 neutrons others 118 or
119 neutrons, 120 is a nice average).
A water molecule consists of two hydrogen atoms with 1 proton each and
an oxygen atom with 8 protons and 8 neutrons. That gives an atomic
weight of 18.
If you divide 200 by 18 you get 11.1
That is not quite 13.6, so there must be a few more mercury atoms
squeezed into a volume than you would find in water. About 20%
more mercury atoms per cubic centimeter is about all you would need.









