Thursday Mar. 23, 2006
The Experiment #3 reports and the revised Expt. #2 reports were
collected today (some people may have turned them in last Tuesday)
All of the Experiment #4 materials have been checked out.
There
are now plenty of Expt. 3 materials available, however. If you
have not done a book, scientific paper, or experiment report you should
check out an Experiment #3 kit and perform that experiment.
All of the Assignment #2 1S1P reports have been graded.
We have a
few more humidity sample problems to work through.
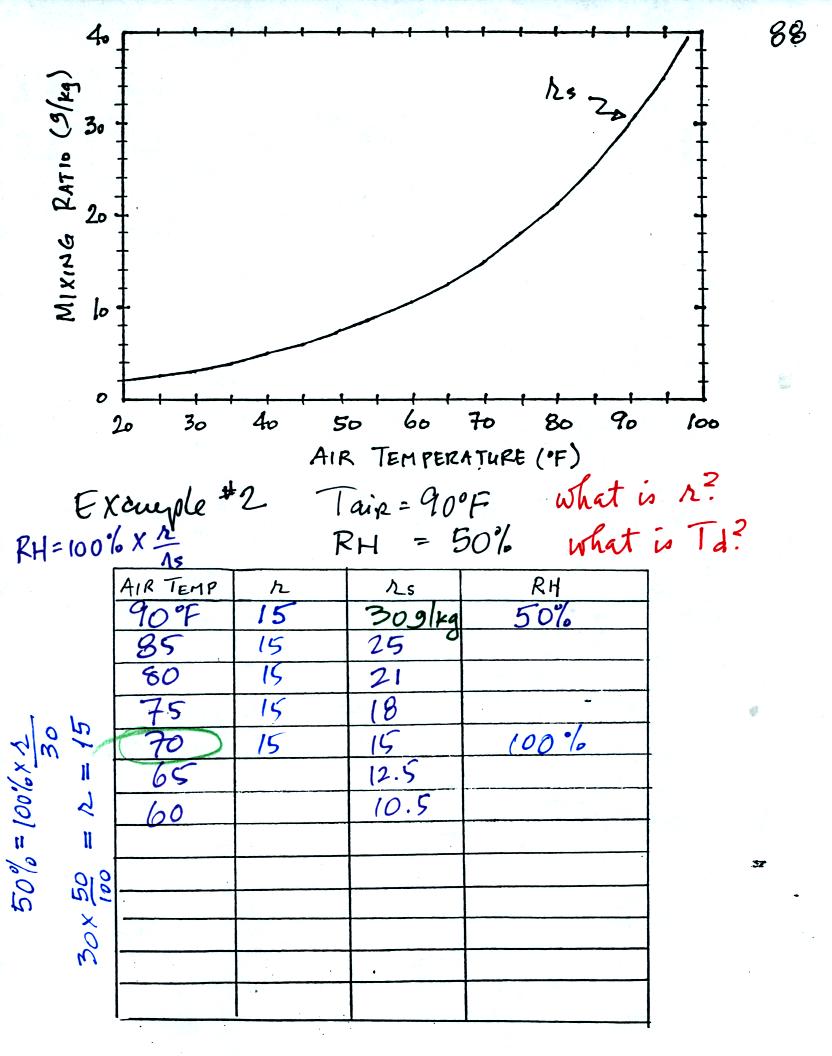
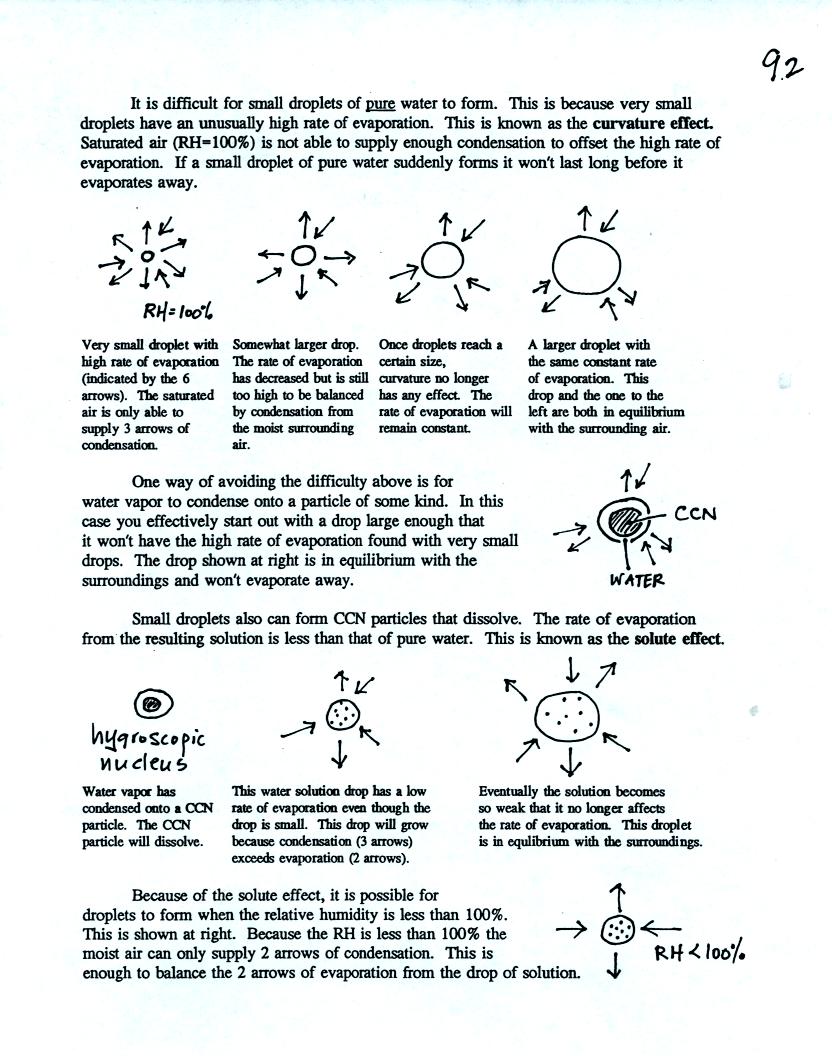
Here is the second sample problem. Given an air
temperature of 90
F and a relative humidity of 50% you are supposed to figure out the
mixing ratio (15 g/kg) and the dew point temperature (70 F). The
problem is worked out in detail below.
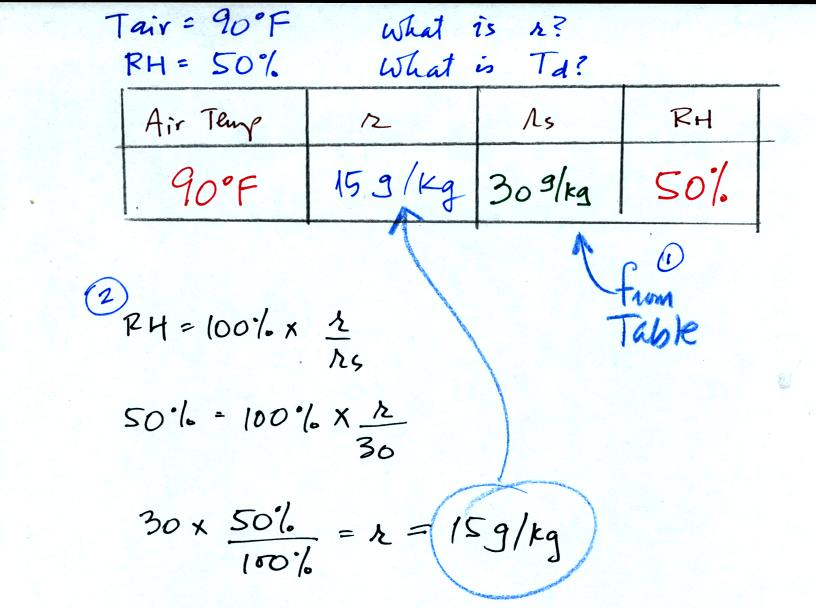
First you fill in the air temperature and the RH data that
you are
given. Since you know the air's temperature you can look up the
saturation mixing ratio (30 g/kg). Then you can substitute into
the relative humidity formula and solve for the mixing ratio (15 g/kg).
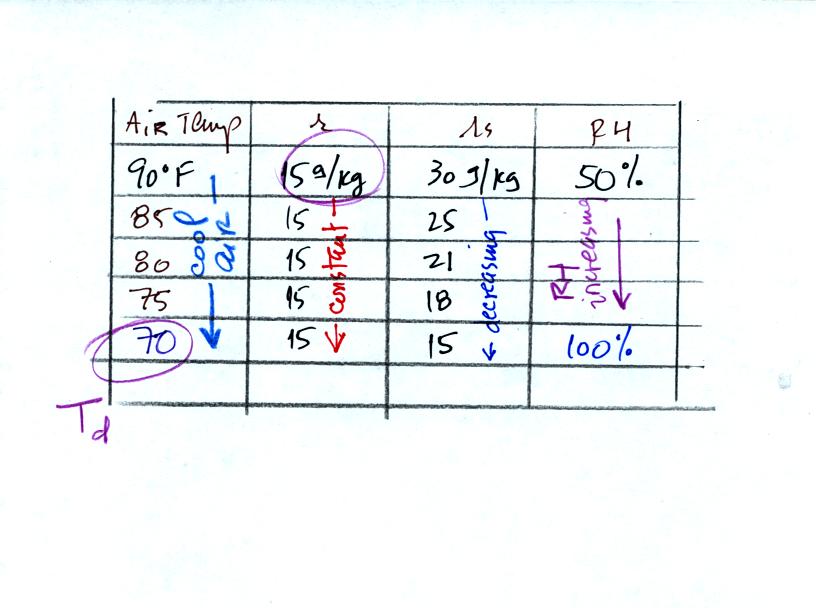
Finally you imagine cooling the air. Cooling causes
the
saturation mixing ratio to decrease, the mixing ratio stays constant,
and the relative humidity increases. In this example the RH
reached 100% when the air had cooled to 70 F. That is the dew
point temperature.

Something new you can learn from Examples 1 and 2. In
the first
example the RH was low and the difference between the air and dew point
temperatures was large. In the second example, the RH was higher
and the difference between the air and dew point temperatures was
smaller. If there were no difference between the air and dew
point temperature the RH would be 100%.
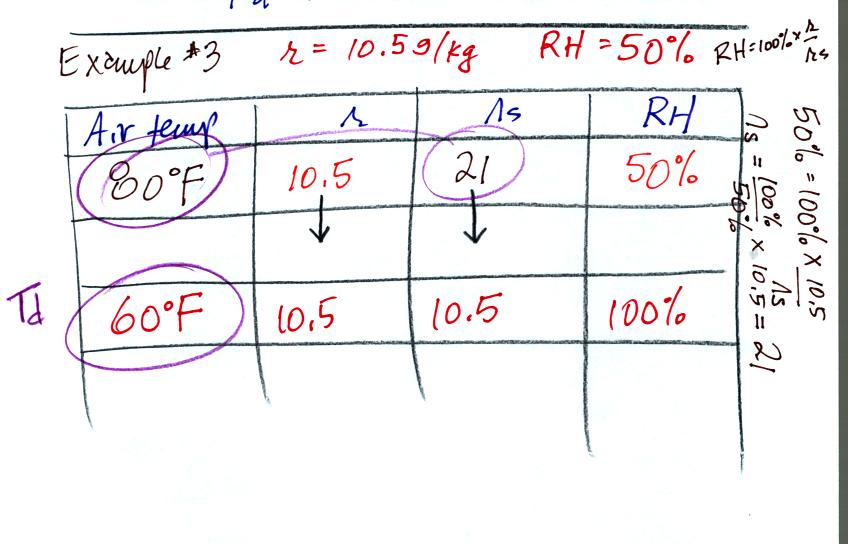
In the 3rd problem we started with RH=50% and r=10.5
g/kg.
The air contains 10.5 g/kg of water vapor, this is 50% of what the air
could potentially hold. So the air's capacity, the saturation
mixing ratio must be 21 g/kg. Once you know the saturation mixing
ratio you can look up the air temperature in a table. Then you
imagine cooling the air until the RH becomes 100%. This occurs at
60 F. The dew point is 60 F.
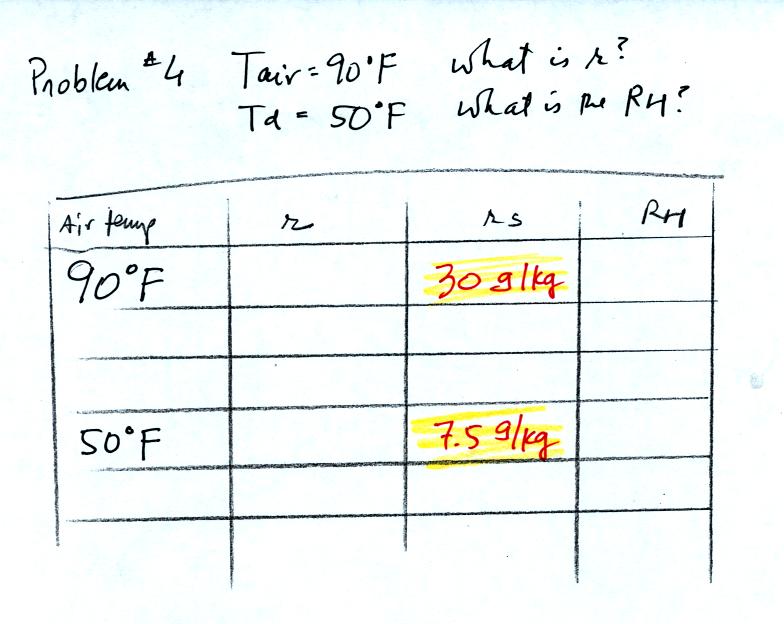
In the last example problem we start with an air temperature
of 90 F
and a dew point temperature of 50 F.
The first thing you can do is look up the saturation mixing ratio for
90 F and 50 F air.
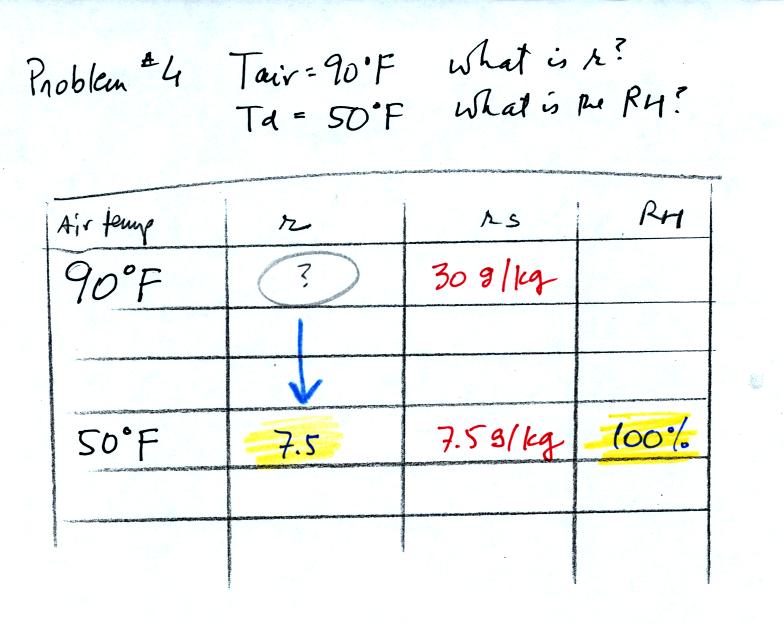
Then we know that if we cool the 90 F air to 50 F the RH
will become
100%. Since we know the saturation mixing ratio value at 50 F is
7.5 g/kg we can say the mixing ratio is 7.5 g/kg.
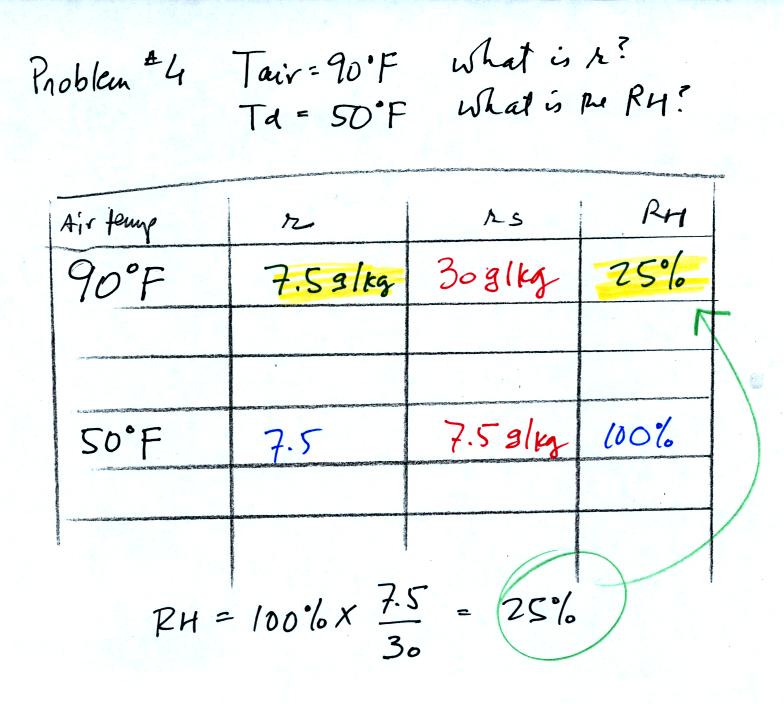
Now that we know the mixing ratio we can compute the
relative humidity.
Dew point temperature and mixing ratio both give you an idea of the
actual amount of water vapor in the air. If you know the mixing
ratio you can determine the dew point temperature. If you know
the dew point temperature you can determine the mixing ratio (that's
what we did in this problem).
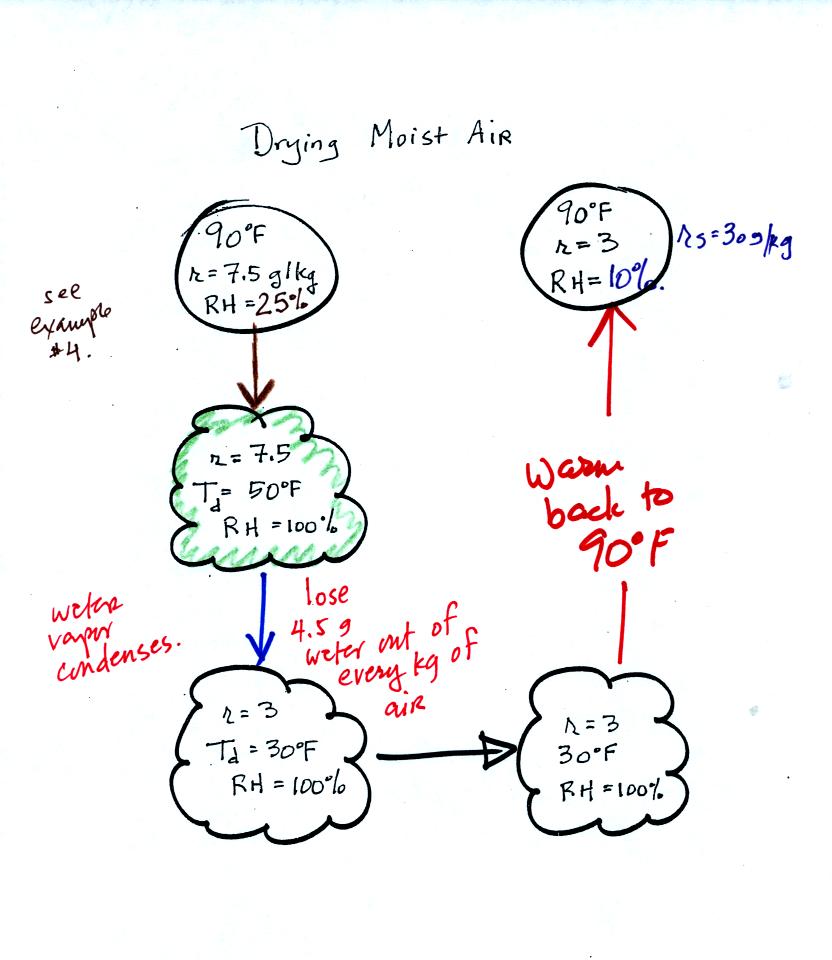
90 F air with 25% relative
humidity is cooled to 50 F the dew point. The relative humidity
reaches 100% at that point. Then the air is cooled some more, to
30 F. The 30 F air can't hold the 7.5 g/kg of water vapor that
was found in the 50 F air. Some of the moisture must
condense. Next the 30 F air now containing only 3 g/kg of water
vapor is warmed to 90 F, the starting temperature. The air now
has a RH of only 10%. The air contains less than half the
moisture it did originally.

This figure was not
shown in
class. Cooling moist air below the dew point is kind of
like squeezing out or wringing out a wet sponge. You start to
squeeze the sponge until water starts to drop out and then squeeze it
some more. Then you let go of the sponge and let it expand back
to its orignal shape and size. The sponge will be drier than when
you started.
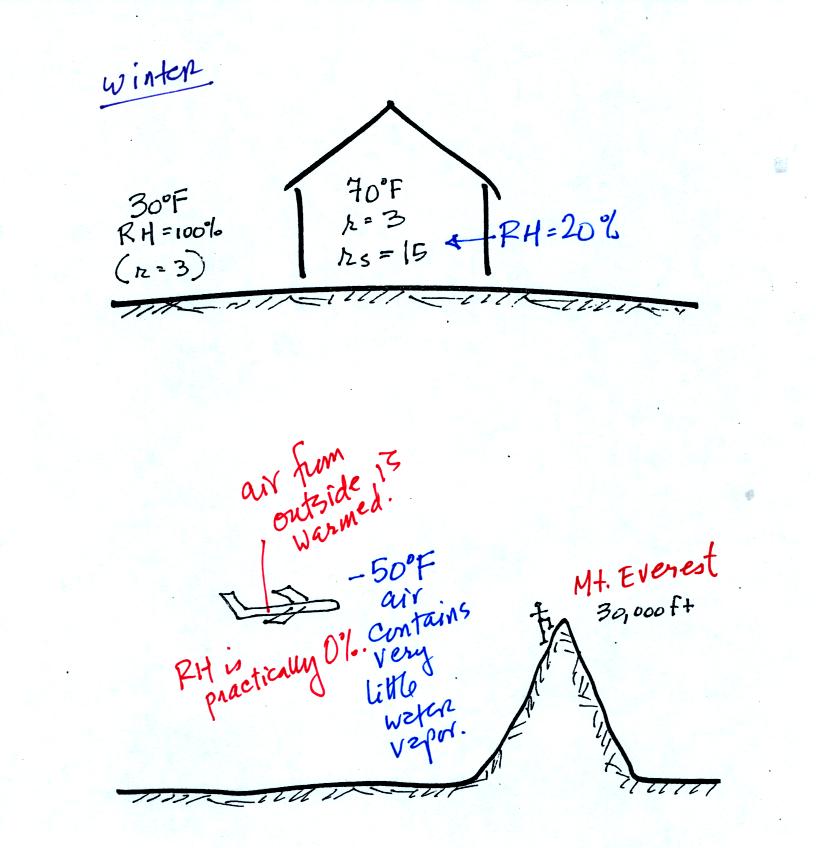
These two figures show where this kind of thing can
occur. In the
winter cold air is brought inside your house or apartment and
warmed. Imagine 30 F air with a RH of 100 % brought inside and
warmed to 70 F. The RH will decrease to 20%.
The air in an
airplane comes from outside the plane. The air outside the plane
is very cold (-50 F perhaps) and contains very little water
vapor. When brought inside and warmed to a comfortable
temperature the RH of the air in the plane will be very close 0%.
Actually the ventilation system in the plane will add moisture to the
air so that it doesn't get that dry.
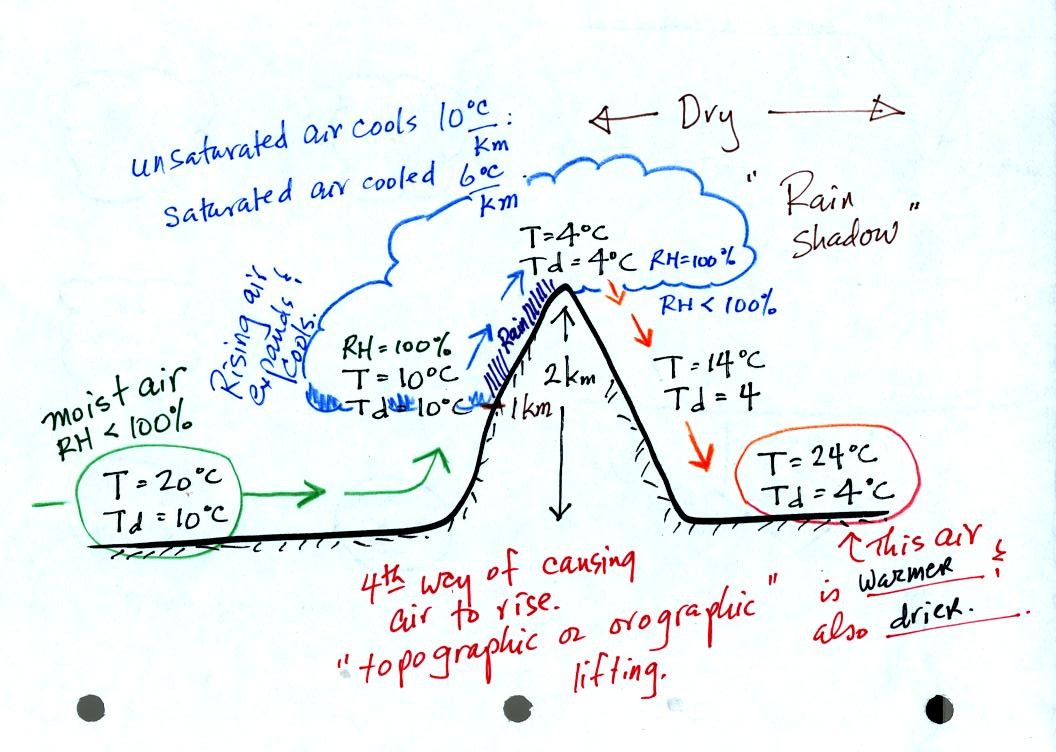
Here's an important cooling and drying moist air example. Moist air
traveling up and over a mountain cools to its dew point
temperature at 1 km altitude. A cloud forms at that point.
The parcel of air continues to rise and cool. This additional
cooling below the dew point "squeezes" moisture out of the air.
The moisture falls to the ground as rain. Note that rising
unsaturated air cools at a rate of 10 C/km between 0 and 1 km altitude,
saturated air cools at 6 C/km (release of latent heat in the rising air
during condensation partially offsets the cooling due to expansion)
between 1 and 2 km altitude.
The air travels back down the right side of the mountain and
warms. Note the air ends up warmer (24 C vs 20 C) and drier (Td =
4 C vs Td = 6 C) than when it started out. The downwind side of
the mountain is referred to as a "rain shadow" because rain is less
likely there than on the upwind side of the mountain.
Note topographic or orographic lifting is a 4th way of causing air to
rise (convergence into low pressure, convection, and fronts are the
other 3 ways). Rising air is important because rising air expands
and cools. When you cool moist air the RH increases. The RH
may increase enough (if you cool the air to the dew point temperature)
that a cloud can form.
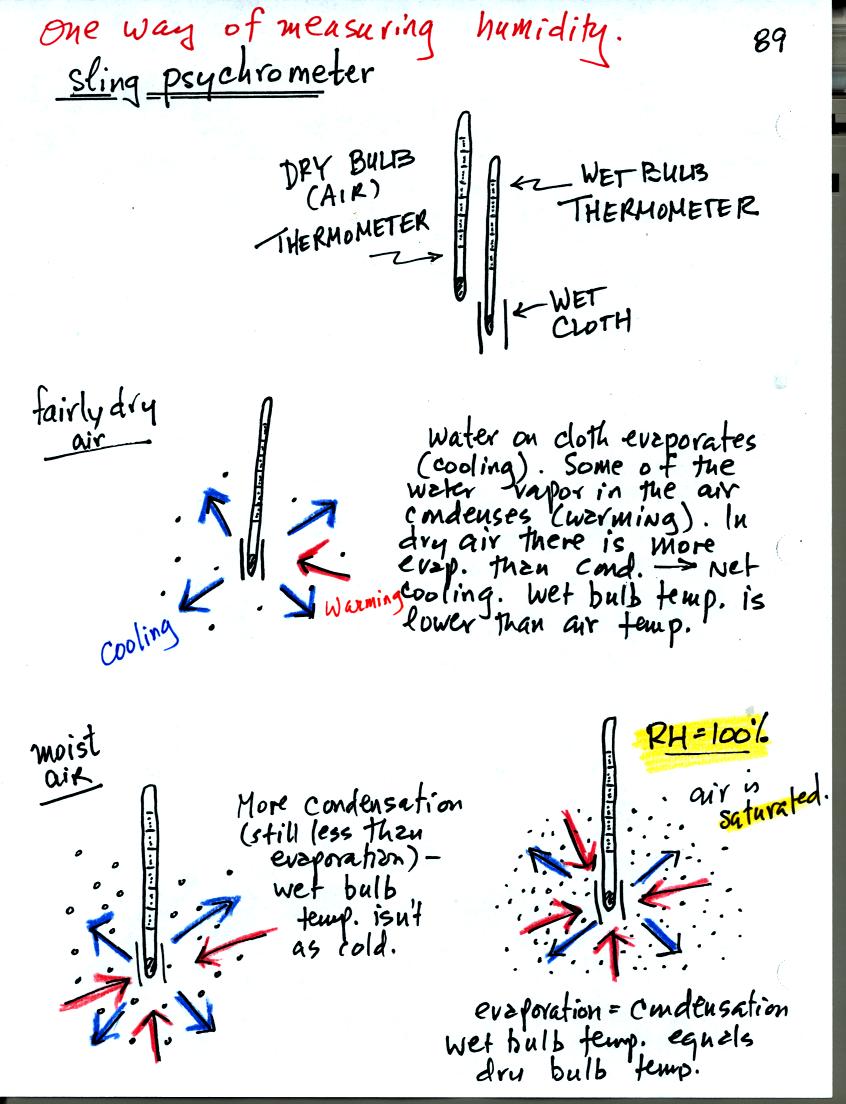
You can use tabulated data (see Appendix D in your textbook) and
measurements made with a sling psychrometer to measure the dew point
and relative humidity. A sling psychrometer consists of two
thermometers mounted side by side. One of the thermometers is
covered with a piece of cloth that is moistened with water. As
the thermometers are swung through the air, water will evaporate from
the wet thermometer. Evaporation is a cooling process. At
the same time some of the water vapor from the surrounding air will
condense onto the thermometer and warm it. The temperature
difference between the two thermometers provides a measure of the
amount of moisture in the air.

Here's another look at what happens. In the first case, fairly
dry air, the wet thermometer starts out at 70 F. Because there
are 4 arrows of evaporation and only 1 arrow of condensation the
thermometer cools quickly to 45 F. At 45 F the water on the
thermometer evaporates at a slower rate. The one arrow of
evaporation is balanced by 1 arrow of condensation. The wet
thermometer won't cool below 45 F. Note the large, 25 F,
difference between the dry and wet bulb thermometers.
In the second case, moister air, the thermometer starts at 70 F again
with 4 arrows of evaporation. Because the air is moister there
are 3 arrows of condensation. The thermometer cools to 65
F. That lowers the rate of evaporation slightly. Now the
rates of evaporation and condensation are equal. With this
moister air there isn't as much of a difference between the dry and wet
bulb thermometer readings.
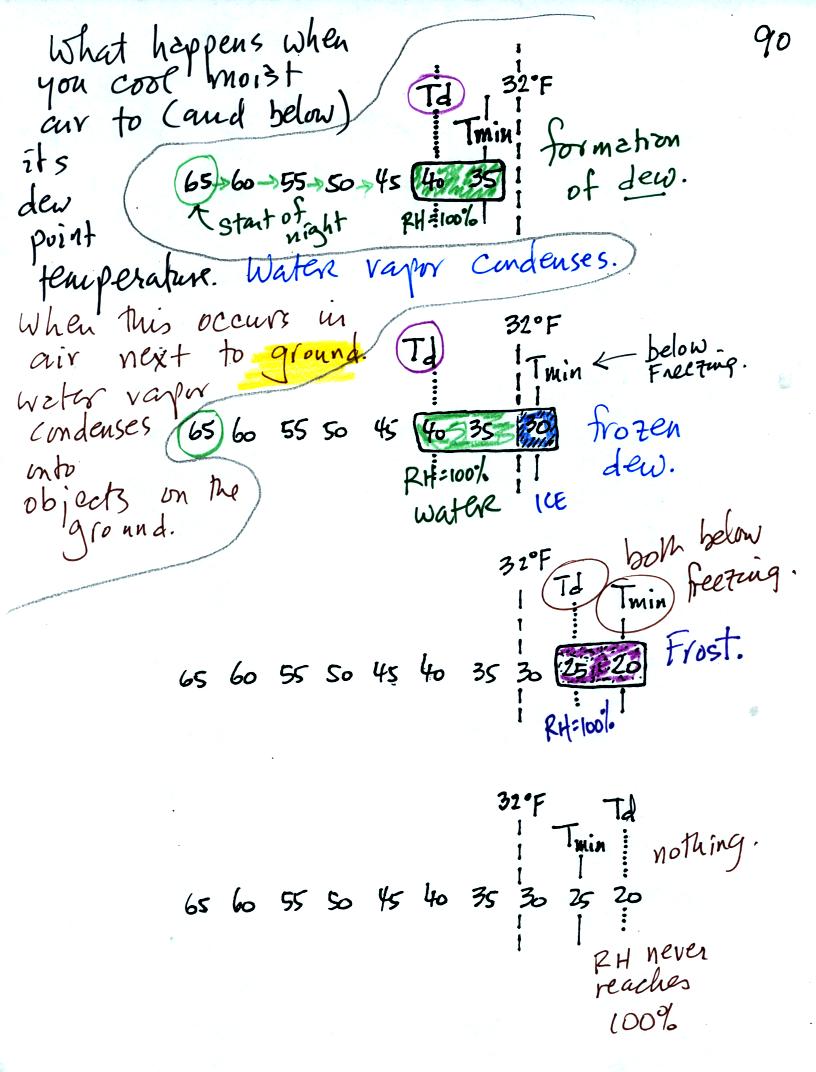
Now we have seen that when you cool air to its dew point (or
below the dew point), water vapor begins to condense out of the
air. When this occurs in air next to the ground the water vapor
condenses onto objects on the ground.
In the first example air that starts out with a temperature of 65 F
at the beginning of the night. It cools to 35 F during the
night. When the air reaches 40 F, the
dew point, the RH reaches 100%. As the air temperature drops
below the dew point and cools to 35 F water vapor will condense onto
the ground or objects on the ground (such as an automobile). This
is dew.
The dew point is the same but the nighttime minimum temperature
is below freezing in the second example. Dew will form when the
air temperature reaches 40 F. Once the air temperature drops
below 32 F the dew will freeze and form frozen dew.
In the third example both the dew point and nighttime minimum
temperatures are below freezing. When the air temperature drops
below the dew point, water vapor turns directly to ice (deposition) and
forms frost.
The dew point in this case is sometimes called the frost point.
The air never becomes saturated in the fourth example because the
nighttime minimum temperature never cools to the dew point.

When air above the ground becomes saturated the water vapor
condenses
onto small particles in the air called cloud condensation nuclei
(CCN). With some hygroscopic materials condensation begins when
the RH is less than 100% (see p. 92, reproduced at the end of today's
notes, for more explanation of why this occurs).
A short video showing how water vapor would, over time,
preferentially
condense onto small grains of salt rather than small spheres of glass.
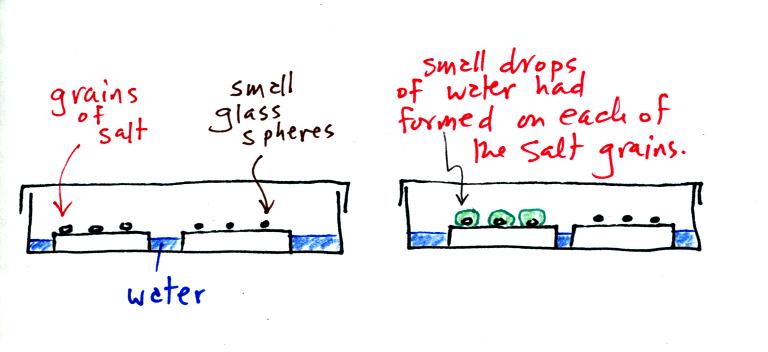
The start of the video at left showed the small grains of
salt were
placed on a platform in a petri dish
containing water. Some small spheres of glass were placed in the
same
dish. After about 1 hour small drops of water had formed around
each
of the grains of salt (shown above at right).
Here's
some more information about the formation of cloud droplets. This wasn't covered in class and
you do not need to worry about learning about these details. This
is one of those "for further reading" sections.