Friday Feb. 16, 2007
The graded quizzes were returned in class today. Please check
your quiz carefully for errors.
You can
check the progress being made grading the 1S1P Assignment #1 reports at
this link.
Here's an example of a grade that you might see when you get your 1S1P
report.

The first number refers to the information content of your
report. You could earn up to 8 pts for content on Topic #1 (The
Earth's Changing Climate) reports (the only ones graded so far).
The more specfic details and
information that you include in your report the higher this grade will
be. The second number is the quality of the writing. To
receive a high writing grade your report should be your understanding
and explanation of the material (it should be written in your words,
you should just paraphrase the discussion in the textbook), should be
free of spelling mistakes
and grammatical errors. The person above received 11.5 out of a
total 15 pts possible. Don't think of this as a percentage
grade. You can earn up to 45 1S1P pts during the semester.
The person above has 45 - 11.5 = 33.5 pts left to go. As long as
this
person keeps writing and turning in reports in future assignments he or
she
shouldn't have any trouble reaching the 45 pts goal.
First
sometime the depends on specific heat, something we covered on
Monday. The
surface of the earth is largely made up of
water (oceans) and soil. Water has a larger specific heat (heat
capacity) than soil. Oceans are slow to warm in the summer and
slow to cool during the winter.
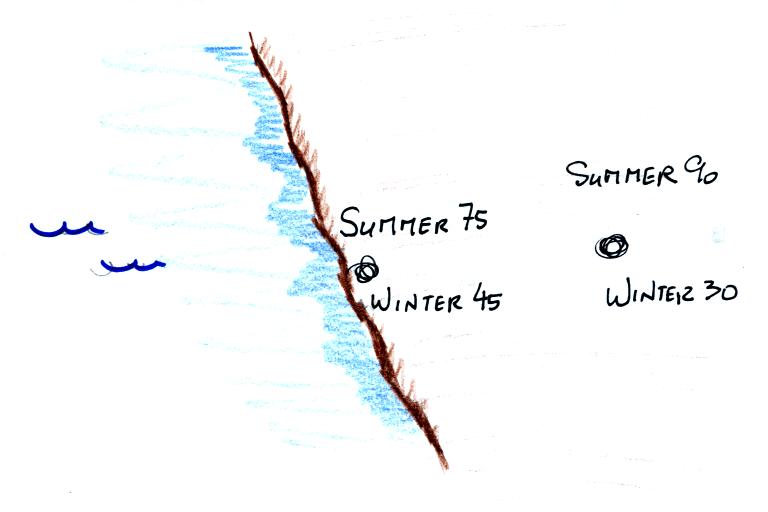
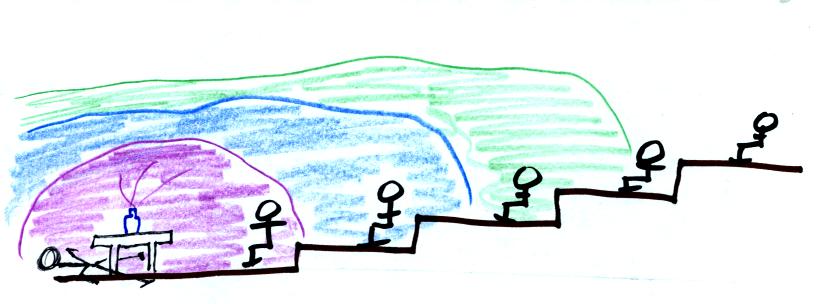
Oceans moderate the climate. The city above on the
coast has a 30o F annual range of temperature. The
city further
inland (assumed to be at the same latitude and altitude) has an annual
range of 60o F. Note that both cities have the same 60o
F annual
mean temperature. Proximity to land or water is one of three or
four factors that determine a region's climate. Latitude and
altitude also play important roles. This is discussed in Chapter
3 and will probably be a topic on a future 1S1P assignment.
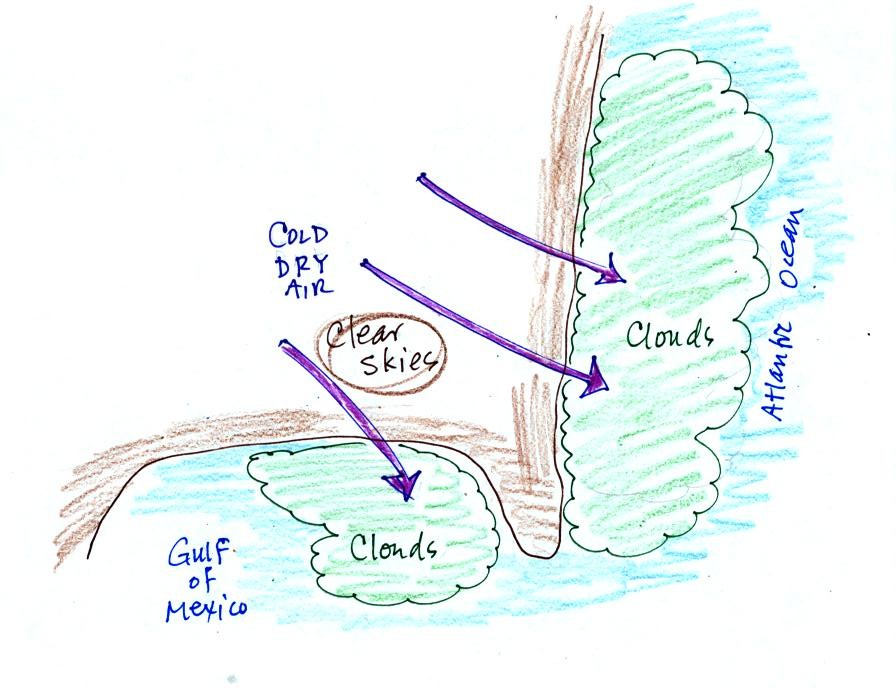
A very
cold and dry air mass has moved over the eastern portion of the United
States. We saw on a satellite photograph that showed as this air
moved
off land and out over the Gulf of Mexico and the Atlantic Ocean a thick
layer of low level clouds formed.
This is a pretty good example of a phenomenon called the "Lake
Effect." The Lake Effect was in the news a week or so also.
Cold dry air blowing across the Great Lakes dumped upwards of 10 feet
of snow on parts of New York.
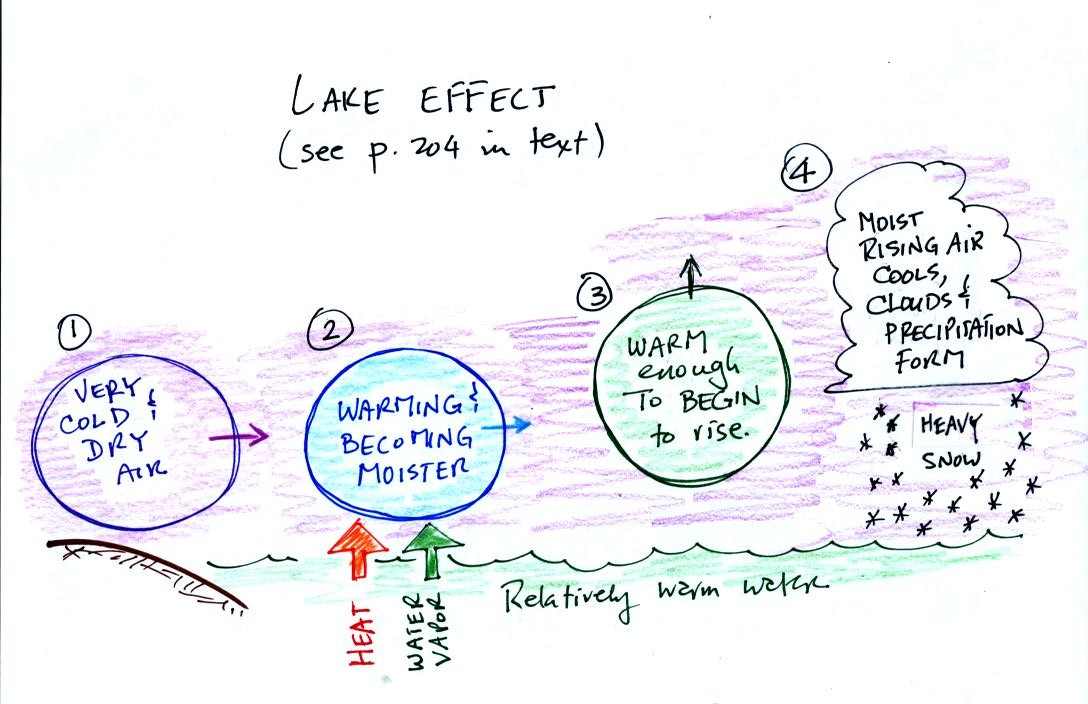
You wouldn't expect cold dry air to produce record amounts of snow and
it doesn't. The cold dry air is modified as it moves over warmer
lake or ocean water. Heat and moisture from the water flow into
the cold dry air mass. The heat warms the air and makes it
bouyant. If the rising air is moist enough and
cools enough, clouds and precipitation can result.
If you add
energy to a material it will usually warm.
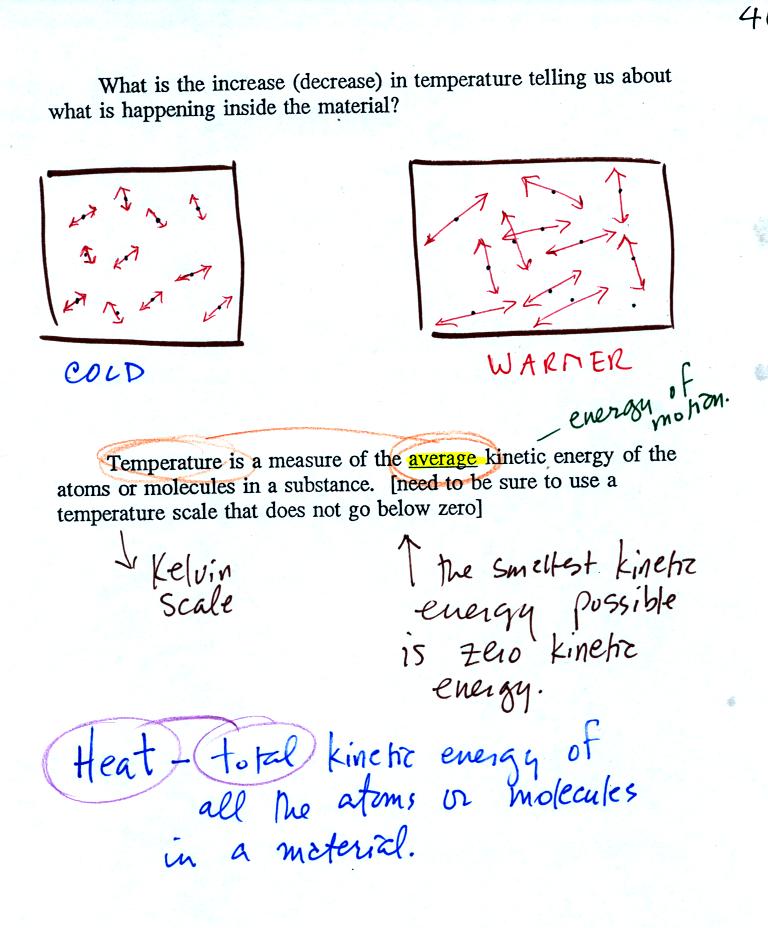
Temperature provides a measure of the average kinetic of the
atoms or
molecules in a material.
You can think of heat as being the total kinetic energy of all
the
molecules or atoms in a material.
The next figure might make the distinction between temperature (average
kinetic energy) and heat (total kinetic energy) clearer.
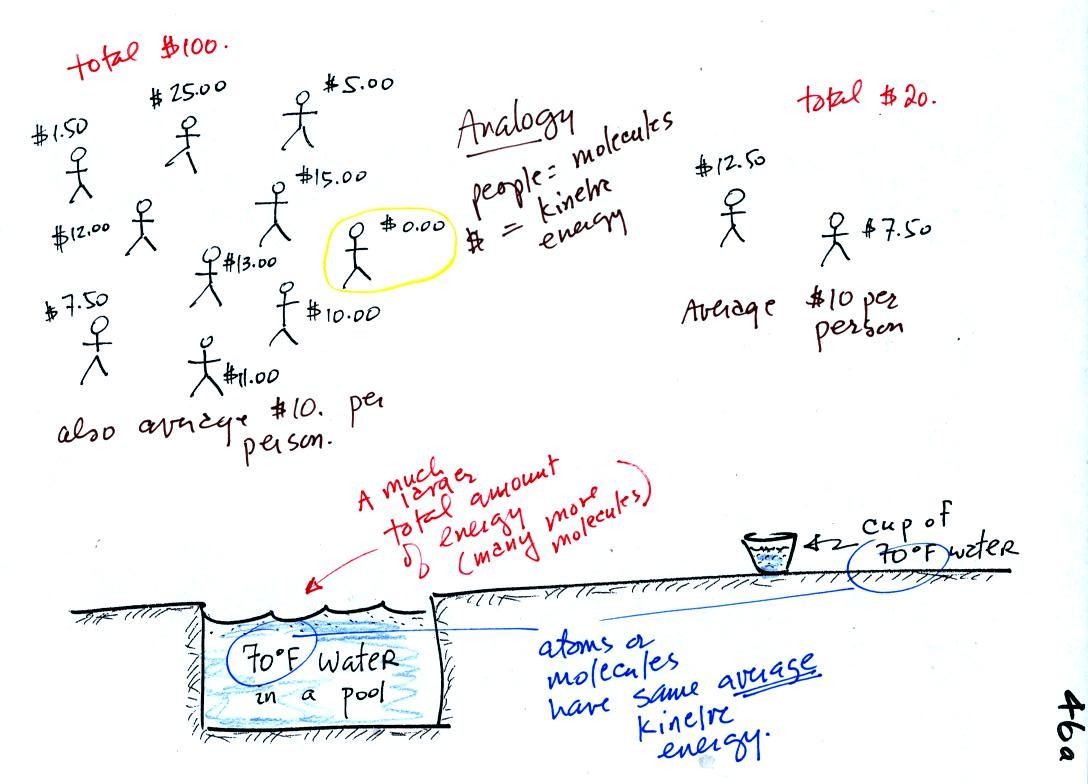
A cup of water and a pool of water both have the same
temperature. The average kinetic energy of the water molecules in
the pool and in the cup are the same. There are a lot more
molecules in the pool than in the cup. So if you add together all
the kinetic
energies of all the molecules in the pool you are going to get a much
bigger number than if you sum the kinetic energies of the molecules in
the cup. There is
a lot more stored energy in the pool than in the cup. It would be
a lot harder to cool (or warm) all the water in the pool than it would
be the cup.
In the same way the two groups of people shown have the same average
amount
of money per person. The $100 held by the group at the left is
greater than the $20 total possessed by the smaller group of people on
the right.
You need to be careful what temperature scale you use when using
temperature as a measure of average kinetic energy. You must
use the Kelvin temperature scale because it does not go
below zero. THe smallest kinetic energy you can have is zero
kinetic energy.
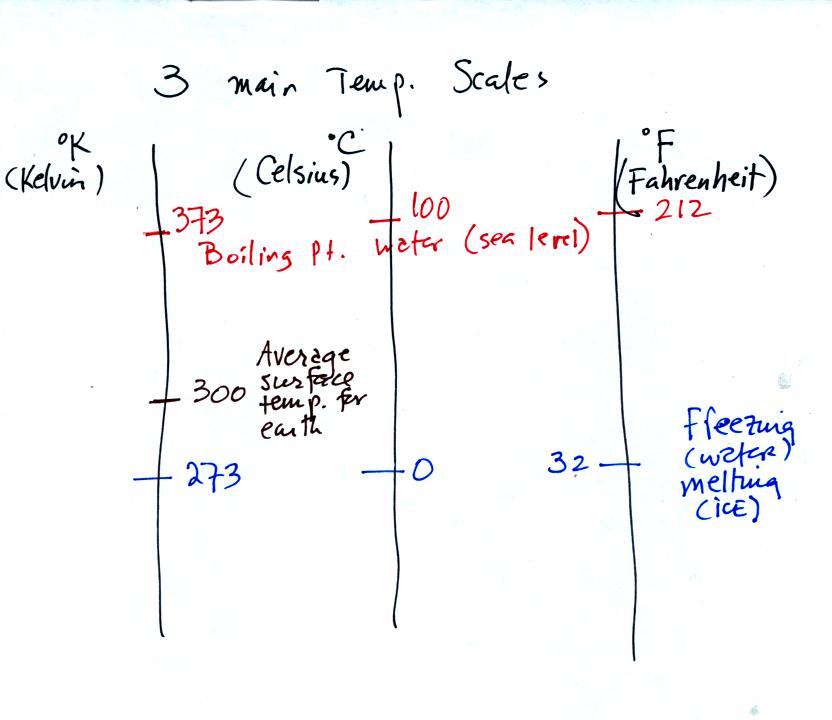
You should remember the temperatures of the boiling point
and freezing
point of water on the Fahrenheit, Celsius, and Kelvin scales. 300
K is a
good easy-to-remember value for the global annual average surface
temperature of the earth.
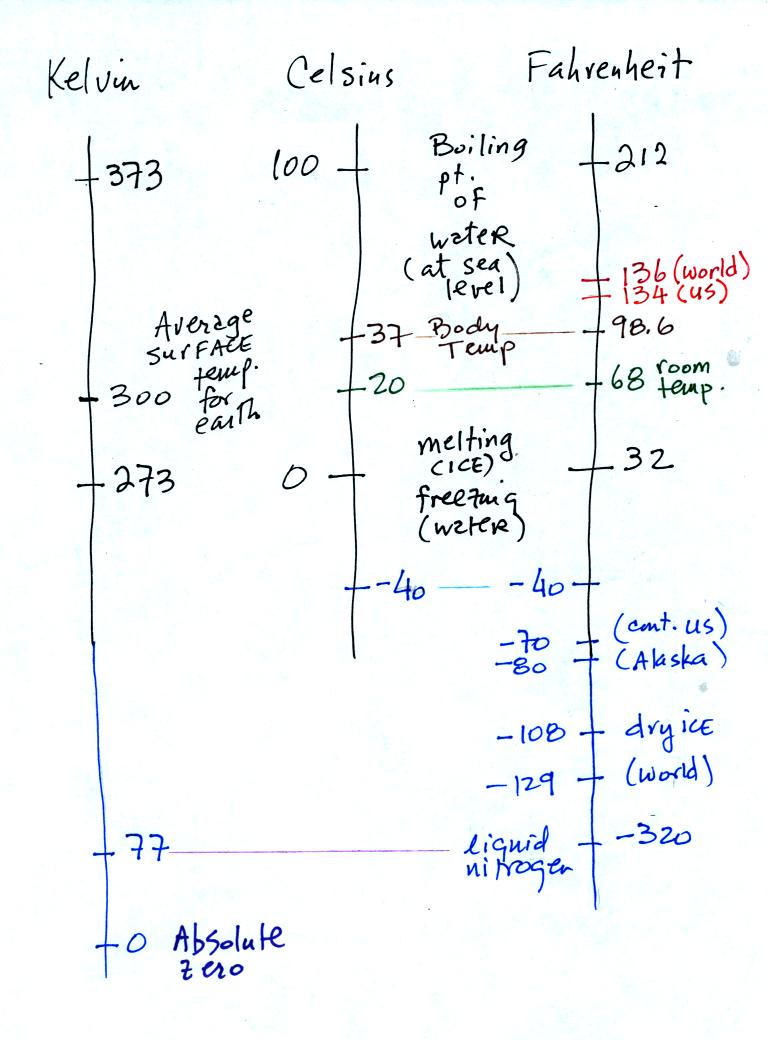
You certainly don't need to try to remember all these
numbers. The world high temperature record was set in Libya, the
US
record in
Death Valley. The continental US cold temperature record of -70 F
was set in Montana and the -80 F value in Alaska. The world
record -129 F was measured at Vostok station in Antarctica. This
unusually cold reading was the result of three factors: high latitude,
high altitude, and location in the middle of land rather than near or
surrounded by ocean. You'll find more record high and low
temperature data on p. 58 and p. 60 in Chapter 3 of the text.
Precipitation records are shown on p. 348. Note that even liquid
nitrogen is still quite a bit warmer than absolute zero.
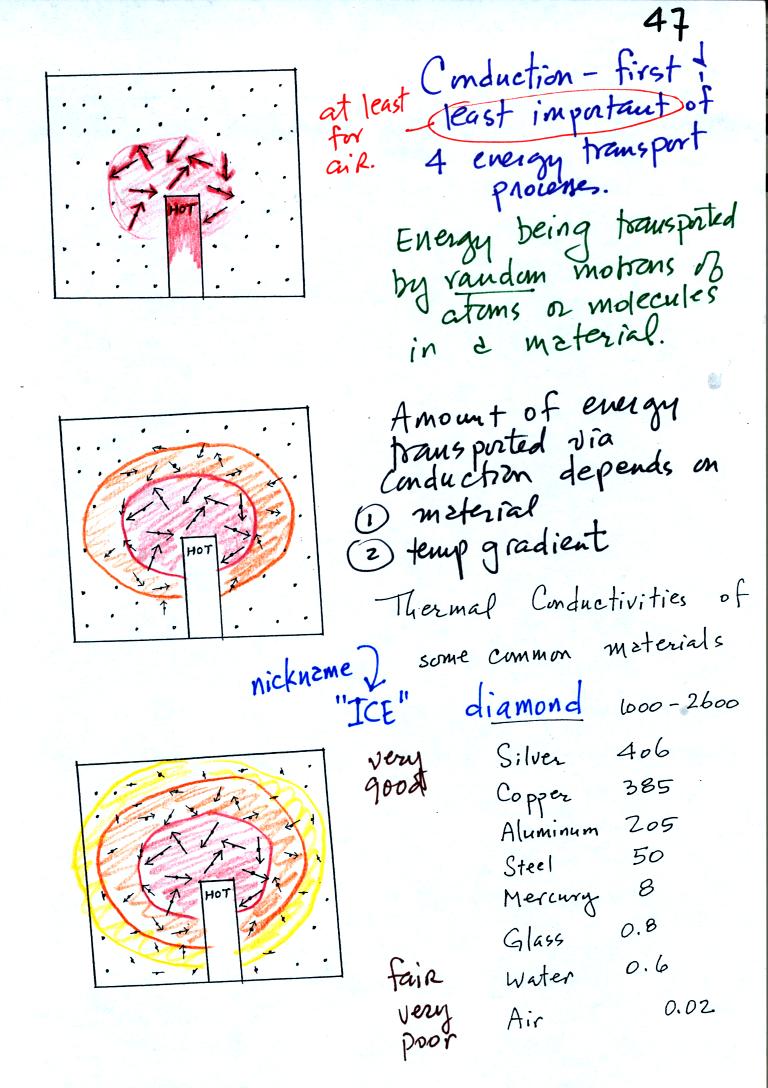
Conduction is the first of four energy transport processes that we
will cover. The figure above illustrates this process. A
hot object is stuck in the middle of some air. In the first picture the
random motions of the atoms or
molecules near the object have caused them to collide with and pick up
energy from the object. This is reflected by the increased speed
of motion or increased kinetic energy of these molecules or
atoms (these guys are colored red). In the middle picture the
initial bunch of
energetic molecules have
collided with some of their neighbors and shared energy with
them (these are orange). The neighbor molecules have gained
energy though they don't
have as much energy as the molecules next to the hot object. In
the third picture molecules further from the object now have gained
some energy (the yellow ones). The random motions and collisions
between molecules
is carrying energy from the hot object out into the colder material.
The rate of energy transport depends on the material. Thermal
conductivities of some materials are listed above. Air is a very
poor conductor of energy. Air is generally regarded as an
insulator. Water is a little bit better conductor. Metals
are generally very good conductors (sauce pans are often made of
stainless steel but have aluminum or copper bottoms to evenly spread
out heat when placed on a stove). Diamond has a very high
thermal conductivity. Diamonds are sometimes called "ice."
They feel cold when you touch them. The cold feeling is due to
the fact that they conduct energy very quickly away from your warm
fingers when you touch them.
The rate of energy transport also depends on temperature
difference. If the object in the picture had been warm rather
than hot, less energy would flow or energy would flow at a slower into
the surrounding material.
The next figure shows a demonstration that we didn't do in
class.
If the instructor were to open a bottle of something with a strong
smell such as glacial acetic acid (acetic
acid gives vinegar its characteristic smell) in the front of the
classroom, the odor would eventually spread throughout the class
room. This is an example of diffusion. The acetic acid
molecules would be moved through the room by random collisions with air
molecules. In many respects this is like the conduction of
heat. The demonstration wasn't performed because the
concentration of the acetic acid in the air, at least in the front of
the room, would be high enough to present a serious risk to the
instructor and students.

The acetic acid is beginning to evaporate into the
air. Collisions with air molecules would begin to move the acetic
acid molecules toward the back of the room.
The instructor has lost consciousness because of the
strong odor of the acetic acid in the front of the room.
The odor would eventually spread throughout the class
room.
Convection
is the next energy transport process we'll look at.

This definition is as far as we got in class. The material below
was added after class. We'll review this quickly in class on
Monday.
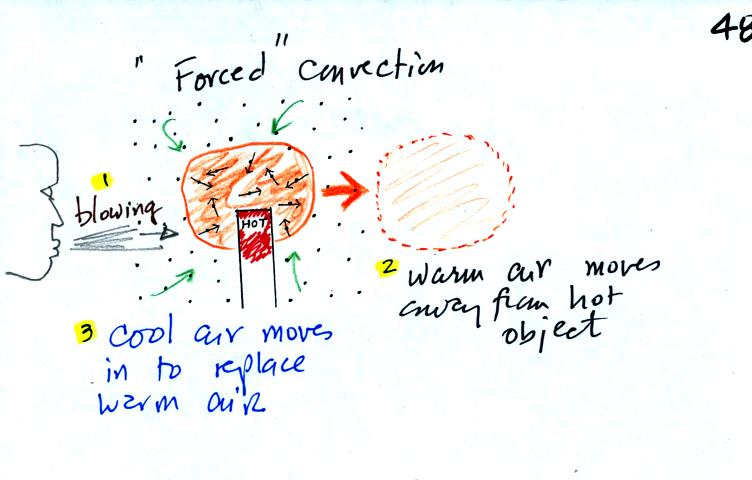
In the picture above the air surrounding a hot object has
been
heated by conduction. Then a person (yes that is a drawing of a
person's head) is blowing the blob of warm air
off to the right. The warm air molecules are moving away from the
hot object together as a group (that's the organized part of the
motion).
Cooler air moves in and surrounds the hot
object and the cycle can repeat itself. This is forced
convection. If you have a hot object in your hand you could just
hold onto it and let it cool by conduction. That might take a
while because air is a poor conductor. Or you could blow on the
hot object and force it to cool more quickly.
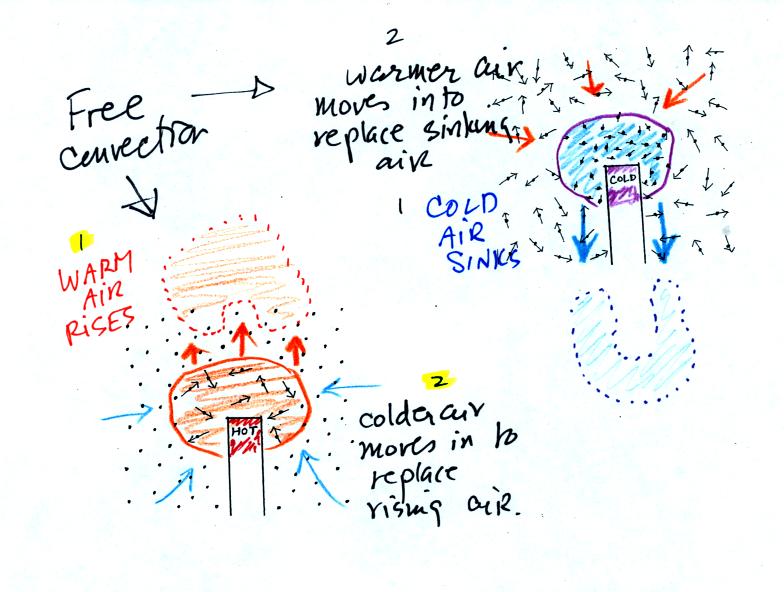
Note in the figure above (at lower left) that, because hot air is also
low density, it actually isn't necessary to blow on the hot object, the
warm air will rise by itself. Energy is being transported away
from the hot object into the cooler surrounding air. This is
called free convection and
represents another way of causing rising air motions in the atmosphere
(rising air motions are important because rising air expands (as it
moves into lower pressure surroundings) and cools. If the air is
moist, clouds can form.)
Note the example at upper right is also free convection. The
sinking
air motions that would be found around a cold object have the effect of
transporting energy from the warm surroundings to the colder object.














